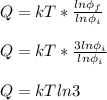Physics, 21.10.2020 16:01 jothianddeepi
Let k be the Boltzmann constant. If the thermodynamic state of gas at temperature T changes isothermally and reversibly to a state with three times the number of microstates as initially, the energy input to gas as heat is:
a. Q = 0
b. Q = 3kT
c. Q = −3kT
d. kT ln 3
e. −kT ln 3

Answers: 2
Another question on Physics

Physics, 22.06.2019 03:30
Scout feels ill one day before school. her mother puts a thermometer in her mouth and the temperature begins to climb to 100°f. what is happening between the cooler thermometer and scout's body? a) scout's body is applying a force to the particles in the thermometer. b) kinetic energy is being transferred from her mouth to the thermometer. c) potential energy is being lost by the thermometer and gained by scout's body. d) the potential energy stored in foods is converted to mechanical energy to raise the mercury in the thermometer.
Answers: 1

Physics, 22.06.2019 06:30
How much force was applied to an object if was moved 2 meters and the work done on the object was 8 joules? a. 0.25 n b. 4 n c. 6 n d. 16 n
Answers: 1

Physics, 22.06.2019 08:00
You have a pick-up truck that weighed 4,000 pounds when it was new. you are modifying it to increase its ground clearance. when you are finished
Answers: 1

Physics, 22.06.2019 11:50
Two resistors r1 and r2 may be connected either in series or parallel across an ideal battery with emf ε. we desire the rate of energy dissipation of the parallel combination to be 8.75 times that of the series combination. if r1 = 105 ω, what are the (a) smaller and (b) larger of the two values of r2 that result in that dissipation rate?
Answers: 2
You know the right answer?
Let k be the Boltzmann constant. If the thermodynamic state of gas at temperature T changes isotherm...
Questions


Health, 22.06.2019 23:00



Business, 22.06.2019 23:00





Health, 22.06.2019 23:00

History, 22.06.2019 23:00

Health, 22.06.2019 23:00


Physics, 22.06.2019 23:00


English, 22.06.2019 23:00

Mathematics, 22.06.2019 23:00