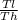Physics, 03.11.2020 17:20 maggiestevens5321
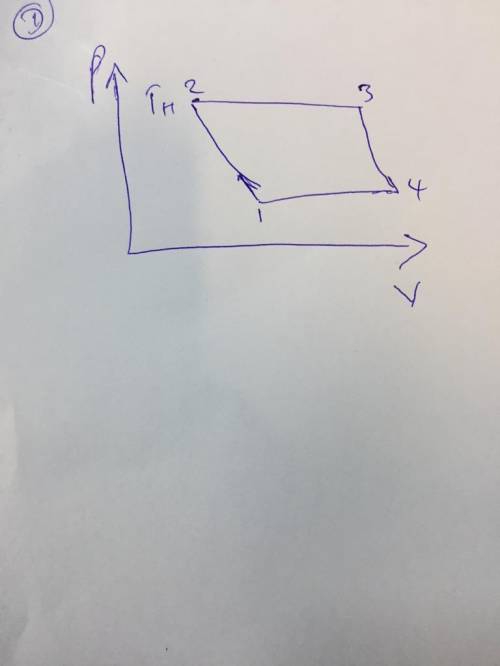
5.54 Two kilograms of air within a piston–cylinder assembly WP execute a Carnot power cycle with maximum and minimum temper atures of 750 K and 300 K, respectively. The heat transfer to the air during the isothermal expansion is 60 kJ. At the end of the isothermal expansion the volume is 0.4 m3. Assuming the ideal gas model for the air, determine a. the thermal efficienc b. the pressure and volume at the beginning of the isothermal expansion, in kPa and m3, respectively. c. the work and heat transfer for each of the four processes, in kJ. d. Sketch the cycle on p–V coordinates.

Answers: 3
Another question on Physics

Physics, 21.06.2019 15:30
Match the atomic particles with their characteristics. 1. made of two protons and two neutrons neutron 2. similar to an electron; positive charge neutrino 3. orbits nucleus; negative charge positron 4. bundle of energy; zero charge proton 5. found in nucleus; positive charge meson 6. very unstable; +, -, or zero charge gamma 7. found in nucleus; zero charge electron 8. negative particle; negative charge beta 9. atomic energy converted to mass alpha
Answers: 1

Physics, 22.06.2019 03:00
Which of the following is not a part of the respiratory system? a. pharynx b. trachea c. pancreas d. larynx
Answers: 1


Physics, 22.06.2019 06:40
How does the kinetic energy of a charge change as the charge moves under the effect of an electric field from higher potential to lower potential? a.the kinetic energy increases.b.the kinetic energy decreases.c.the kinetic energy remains the same.d.the kinetic energy is always zero.
Answers: 1
You know the right answer?
5.54 Two kilograms of air within a piston–cylinder assembly WP execute a Carnot power cycle with max...
Questions



Mathematics, 02.10.2020 14:01








Biology, 02.10.2020 14:01


Social Studies, 02.10.2020 14:01

Business, 02.10.2020 14:01

History, 02.10.2020 14:01




Mathematics, 02.10.2020 14:01