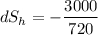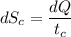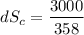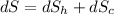When an aluminum bar is connected between a hot reservoir at 720 K and a cold reservoir at 358 K, 3.00 kJ of energy is transferred by heat from the hot reservoir to the cold reservoir. (a) In this irreversible process, calculate the change in entropy of the hot reservoir. J/K
(b) In this irreversible process, calculate the change in entropy of the cold reservoir.
J/K
(c) In this irreversible process, calculate the change in entropy of the Universe, neglecting any change in entropy of the aluminum rod.
J/K
(d) Mathematically, why did the result for the Universe in part (c) have to be positive?

Answers: 3
Another question on Physics

Physics, 21.06.2019 19:30
The us government wants to allocate billions of dollars in the next 10 years to assure our future energy security. the funds will be spread among a variety of possible energy resources. where do you think the government should put the greatest support: solar energy, wind energy, clean coal, oil exploration, gas exploration, or a combination of sources? are there other efforts that should be explored? support your position with cited information for both questions.
Answers: 2


Physics, 22.06.2019 09:00
In a heat engine if 1000 j of heat enters the system the piston does 500 j of work, what is the final internal energy of the system if the initial energy was 2000 j? 1. write the equation 2.list out your known variables 3.plug the numbers into the equations 4.solve 5.write your solution statement that includes initial energy and final
Answers: 1

You know the right answer?
When an aluminum bar is connected between a hot reservoir at 720 K and a cold reservoir at 358 K, 3....
Questions


Physics, 25.02.2021 09:50


Mathematics, 25.02.2021 09:50


Mathematics, 25.02.2021 09:50

Mathematics, 25.02.2021 09:50

Business, 25.02.2021 09:50



Arts, 25.02.2021 09:50



SAT, 25.02.2021 09:50

Social Studies, 25.02.2021 09:50

Mathematics, 25.02.2021 09:50

Computers and Technology, 25.02.2021 09:50

Social Studies, 25.02.2021 09:50


Mathematics, 25.02.2021 14:00

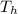 = 720 K
= 720 K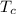 = 358 K
= 358 K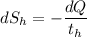 (the negative sign shows the loss of heat)
(the negative sign shows the loss of heat)