
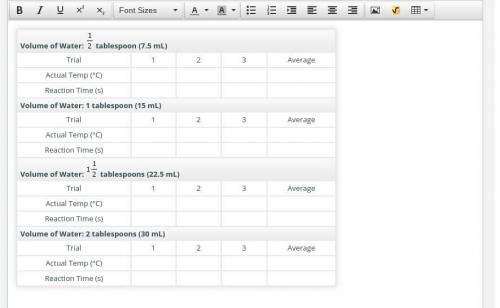
Next, you’ll test your hypothesis by examining the reaction times of vinegar and baking soda in boiling water using four different concentrations of the reactants. You’ll vary the concentrations by using tablespoon (7.5 milliliters), 1 tablespoon (15 milliliters), tablespoons (22.5 milliliters), and 2 tablespoons (30 milliliters) of water. Note that you will use the same amounts of vinegar and baking soda for each trial.
Gather all the materials, and proceed with these steps:
Boil at least 1 cup (240 milliliters) of water on a stove, on a hot plate, or in a microwave oven.
Measure and record the actual temperature of the water.
Measure the required amount of boiling water into the cup.
Add teaspoon (1.5 grams) baking soda to the water, and stir until it is dissolved. The resulting solution will be clear.
Measure out 1 tablespoon (15 milliliters) of vinegar, but do not pour it into the cup yet.
Very quickly, do all of the following:
a. Pour the measured vinegar into the cup.
b. Start the stopwatch.
c. Stir or carefully swirl the substances in the cup.
The chemical reaction will produce bubbles. You’ll be able to see the bubbles and hear them pop. Watch and listen for when the reaction stops. When it looks and sounds like it has finished, stop the stopwatch.
Record the reaction time in the data table.
Discard the solution down the drain, and rinse the cup.
Repeat this procedure, doing three trials at each concentration. Record the average temperature and reaction time for each set of the three trials. Read this math review for a refresher on finding averages.

Answers: 1
Another question on Physics

Physics, 21.06.2019 14:30
Apositive point charge q is fixed on a very large horizontal frictionless tabletop. a second positive point charge q is released from rest near the stationary charge and is free to move. which statement best describes the motion of q after it is released? assume q stays on the table
Answers: 1

Physics, 22.06.2019 13:20
It is reasonable to assume that the bulk modulus of blood is about the same as that of water (2.2 gpa). as one goes deeper and deeper in the ocean, the pressure increases by 10000 pa for every meter below the surface. if a diver goes down 80.0 m in the ocean, by how much does each cubic centimeter of her blood change in volume? give the answer in cubic centimeters (actually one cubic centimeter equals one milliliter).
Answers: 2

Physics, 22.06.2019 15:50
Ryan is examining the energy of the particles in a bar of gold. what is ryan most likely studying?
Answers: 2

Physics, 22.06.2019 18:30
En un laboratorio, nakisha mezcla una solución de hidróxido de sodio con un indicador llamado fenolftaleína. cuando se combinan, crean una solución de color rosa. nakisha se pregunta si la mezcla de otras soluciones con fenolftaleína también creará este color rosa. ? cómo podría nakisha utilizar el proceso de investigación científica para determinar si la mezcla de otras soluciones con fenolftaleína también creará un color rosa? marque las que correspondan.
Answers: 2
You know the right answer?
Next, you’ll test your hypothesis by examining the reaction times of vinegar and baking soda in boil...
Questions

Social Studies, 06.08.2021 02:10



Mathematics, 06.08.2021 02:10





History, 06.08.2021 02:40

English, 06.08.2021 02:40


Mathematics, 06.08.2021 02:40

Biology, 06.08.2021 02:40










