Please Help!
Modeling Radioactive Decay
Directions: For this assignment, you will investigate...

Please Help!
Modeling Radioactive Decay
Directions: For this assignment, you will investigate radiometric dating methods, calculate radioactive isotopes of carbon-14, and
create a graph. As you complete these tasks, be sure to follow all guidelines and instructions.
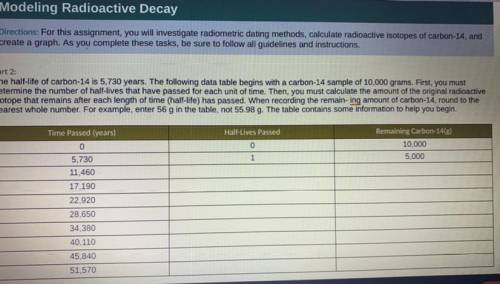
Part 2:
The half-life of carbon-14 is 5,730 years. The following data table begins with a carbon-14 sample of 10,000 grams. First, you must
determine the number of half-lives that have passed for each unit of time. Then, you must calculate the amount of the original radioactive
isotope that remains after each length of time (half-life) has passed. When recording the remaining amount of carbon-14, round to the
nearest whole number. For example, enter 56 g in the table, not 55.98 g. The table contains some information to help you begin.

Answers: 2
Another question on Physics

Physics, 22.06.2019 01:10
Aparticle initially moving east with a speed of 20.0 m/s, experiences an acceleration of 3.95 m/s, north for a time of 8.00 s. what was the speed of the particle after this acceleration, in units of m/s? give the answer as a positive number.
Answers: 1

Physics, 22.06.2019 10:00
Your town is considering building a biodiesel power plant describe at least two advantages and two disadvantages
Answers: 1

Physics, 22.06.2019 16:30
The ph is the a.independent variable b.the dependent variable c.control group
Answers: 1

Physics, 22.06.2019 18:30
What would people living along the coast in south florida do if there was a hurricane warning? move to locations away from the water flock along coasts to watch the natural phenomenon buy instruments to predict the exact location of the hurricane measure water levels to know the exact time of the hurricane
Answers: 3
You know the right answer?
Questions











Social Studies, 25.10.2019 17:43


Social Studies, 25.10.2019 17:43



Physics, 25.10.2019 17:43


Biology, 25.10.2019 17:43




