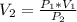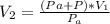Physics, 03.12.2020 17:00 juanitarodriguez
An air bubble released by a deep-water diver, 115 m below the surface of a lake, has a volume of 1.60 cm3. The surface of the lake is at sea level, and the density of the lake water can be approximated as that of pure water. As the bubble rises to the surface, the temperature of the water and the number of air molecules in the bubble can each be approximated as constant. Find the volume (in cm3) of the bubble just before it pops at the surface of the lake. ___ cm3.

Answers: 1
Another question on Physics

Physics, 21.06.2019 15:30
Which of the following statements are true of solids? a.the particles do not vibrate. b.the particles are in a fixed location. c.they have strong intermolecular forces between the atoms or molecules. d.the particles have less kinetic energy than those of liquids or gases.
Answers: 1

Physics, 21.06.2019 19:10
Athin, square metal plate measures 14 cm on each side and has emissivity of 0.60. the plate is heated to a temperature of 745°c. what is the rate at which the plate radiates energy ? the stefan-boltzmann constant is 5.67 × 10-8 w/(m2 ? k4). remember that the plate will radiate energy from both its top and bottom surfaces.
Answers: 1

Physics, 21.06.2019 21:30
Apendulum has a mass of 1.5 kg and starts at a height of 0.4 m. if it is released from rest, how fast is it going when it reaches the lowest point of its path? acceleration due to gravity is g = 9.8 m/s2. a. 2.8 m/s b. 0 m/s c. 5.9 m/s d. 4.3 m/s
Answers: 1

You know the right answer?
An air bubble released by a deep-water diver, 115 m below the surface of a lake, has a volume of 1.6...
Questions

Mathematics, 21.01.2021 06:10

Computers and Technology, 21.01.2021 06:10

Social Studies, 21.01.2021 06:10

History, 21.01.2021 06:10

Social Studies, 21.01.2021 06:10

Biology, 21.01.2021 06:10

Mathematics, 21.01.2021 06:10

Mathematics, 21.01.2021 06:10

Mathematics, 21.01.2021 06:10



Social Studies, 21.01.2021 06:10


Mathematics, 21.01.2021 06:10

Mathematics, 21.01.2021 06:10

Mathematics, 21.01.2021 06:10


History, 21.01.2021 06:10

English, 21.01.2021 06:10

History, 21.01.2021 06:10

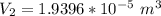
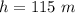
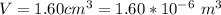
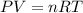
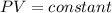
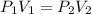
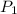 is the pressure of the bubble at the depth where it is released which i mathematically represented as
is the pressure of the bubble at the depth where it is released which i mathematically represented as 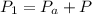
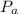 is the atmospheric pressure with value
is the atmospheric pressure with value 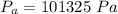
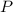 is the pressure due to the depth which is mathematically represented as
is the pressure due to the depth which is mathematically represented as 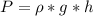
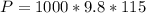
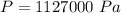
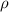 is the density of pure water with value
is the density of pure water with value 
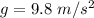
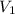 is the volume of the bubble at the depth where it is released
is the volume of the bubble at the depth where it is released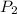 is the pressure of the bubble at the surface which is equivalent to the atmospheric temperature
is the pressure of the bubble at the surface which is equivalent to the atmospheric temperature 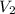 is the volume of the bubble at the surface
is the volume of the bubble at the surface