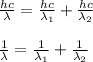A certain atom has only three energy levels. From lowest to highest energy, these levels are denoted n = 1, n = 2, and n = 3. When the atom transitions from the n = 3 level to the n = 2 level, it emits a photon of wavelength 800 nm. When the atom transitions from the n = 2 level to the n = 1 level, it emits a photon of wavelength 200 nm. What is the wavelength of the photon emitted when the atom transitions from the n = 3 level to the n = 1 level?A. 1000 nm
B. 600 nm
C. 500 nm
D. 160 nm

Answers: 1
Another question on Physics

Physics, 22.06.2019 07:40
Astudent creates a model of a closed ecosystem by filling a glass tank half full with water, then adding 10 snails and two small aquatic plants. the next day, all the snails are dead. what is the most likely cause of their death?
Answers: 3

Physics, 22.06.2019 16:00
Apersons beliefs and general outlook, which act like filters on the information they receive are called ?
Answers: 1

Physics, 22.06.2019 17:40
Aball is thrown horizontally from a cliff at 8m/s .in what direction is the ball moving 2s later?
Answers: 1

Physics, 22.06.2019 20:30
Acold front traveling southeast collided with a warm front traveling northwest the following map shows the weather on monday the day the two fronts collided which of these describes the weather forecast for mississippi on tuesday and wednesday
Answers: 2
You know the right answer?
A certain atom has only three energy levels. From lowest to highest energy, these levels are denoted...
Questions

Social Studies, 08.07.2021 06:30

Mathematics, 08.07.2021 06:30


Mathematics, 08.07.2021 06:30

Mathematics, 08.07.2021 06:30



Mathematics, 08.07.2021 06:30





History, 08.07.2021 06:30

Mathematics, 08.07.2021 06:30

Spanish, 08.07.2021 06:30

Mathematics, 08.07.2021 06:30

Mathematics, 08.07.2021 06:30

Mathematics, 08.07.2021 06:30


History, 08.07.2021 06:40