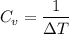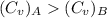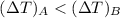Physics, 16.12.2020 16:30 geliyahmoore1
For constant volume processes the heat capacity of gas A is greater than the heat capacity of gas B. We conclude that when they both absorb the same energy as heat at constant volume:
A. the temperature of A increases more than the temperature of B
B. the temperature of B increases more than the temperature of A
C. the internal energy of A increases more than the internal energy of B
D. the internal energy of B increases more than the internal energy of A
E. A does more positive work than B

Answers: 1
Another question on Physics

Physics, 22.06.2019 08:00
What is the average speed of a car that travels 40mph for 1 hour and 60 mph in another hour will mark brainliest
Answers: 1

Physics, 22.06.2019 13:20
Ahanging spring stretches by 35.0 cm when an object of mass 450 g is hung on it at rest. in this situation, we define its position as x = 0. the object is pulled down an additional 18.0 cm and released from rest to oscillate without friction. what is its position x at a moment 84.4 s later? express your answer in cm.
Answers: 1


You know the right answer?
For constant volume processes the heat capacity of gas A is greater than the heat capacity of gas B....
Questions


Mathematics, 14.11.2019 21:31


History, 14.11.2019 21:31

Mathematics, 14.11.2019 21:31

Mathematics, 14.11.2019 21:31


Computers and Technology, 14.11.2019 21:31






History, 14.11.2019 21:31

Social Studies, 14.11.2019 21:31

History, 14.11.2019 21:31

Biology, 14.11.2019 21:31


Mathematics, 14.11.2019 21:31

English, 14.11.2019 21:31

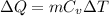
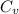 is heat capacity at constant volume
is heat capacity at constant volume