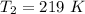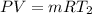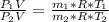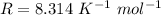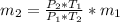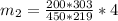Physics, 25.12.2020 17:20 claudioocampo5621
An insulated rigid tank with a volume of 0.57 m3, contains 4 kg of Argon gas at 450 kPa and 30 C. A valve is now opened, and the Argon is slowly allowed to escape until the pressure inside drops to 200 kPa. Assuming the Argon remaining inside the tank has undergone a reversible, adiabatic process, determine the final mass in the tank,

Answers: 2
Another question on Physics

Physics, 21.06.2019 15:50
Electric charge is uniformly distributed inside a nonconducting sphere of radius 0.30 m. the electric field at a point p, which is 0.50 m from the center of the sphere, is 15,000 n/c and is directed radially outward. what is the maximum magnitude of the electric field due to this sphere
Answers: 2

Physics, 22.06.2019 12:00
An architect plans to use solar energy to heat the next house he designs. what principle of absorption and infrared energy can be applied to the design of the new house? how could she apply to those principals?
Answers: 2

Physics, 22.06.2019 14:30
Which of the following bonds would be most polar? a. c-i b. c-br c. c-cl d. c-f e. c-o
Answers: 1

Physics, 22.06.2019 17:00
If you wanted to move an electron from the positive to the negative terminal of the battery, how much work w would you need to do on the electron? enter your answer numerically in joules.
Answers: 1
You know the right answer?
An insulated rigid tank with a volume of 0.57 m3, contains 4 kg of Argon gas at 450 kPa and 30 C. A...
Questions




Mathematics, 16.10.2020 17:01

Mathematics, 16.10.2020 17:01

Mathematics, 16.10.2020 17:01

Mathematics, 16.10.2020 17:01

Mathematics, 16.10.2020 17:01

Mathematics, 16.10.2020 17:01


Mathematics, 16.10.2020 17:01


English, 16.10.2020 17:01

History, 16.10.2020 17:01


Mathematics, 16.10.2020 17:01


Chemistry, 16.10.2020 17:01

Mathematics, 16.10.2020 17:01

Engineering, 16.10.2020 17:01

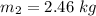
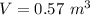
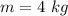
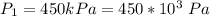
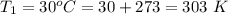
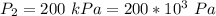
![T_2 = T_1 * [\frac{P_2}{P_1} ]^{ \frac{(k - 1 )}{k} }](/tpl/images/1007/5612/a5d79.png)
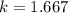
![T_2 = 303 * [\frac{200}{450} ]^{ \frac{(1.667- 1 )}{1.667} }](/tpl/images/1007/5612/8aae2.png)