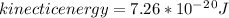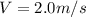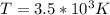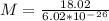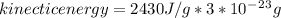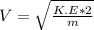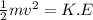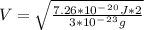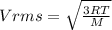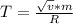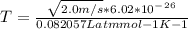The latent heat of vaporization for water at room temperature is 2430 J/g.
1. How much kinetic energy does each water molecule that evaporates possess before it evaporates?
2. Find the pre-evaporation rms speed of a water molecule that is evaporating.
3. What is the effective temperature of these molecules (modeled as if they were already in a thin gas)?
4. Why do these molecules not burn you
a. These molecules got to be slow-moving in collisions that made other molecules fast-moving; the average molecular energy decreases.
b. These molecules got to be slow-moving in collisions that made other molecules fast-moving; the average molecular energy is unaffected.
c. These molecules got to be fast-moving in collisions that made other molecules slow-moving; the average molecular energy is unaffected.
d. These molecules got to be fast-moving in collisions that made other molecules slow-moving; the average molecular energy increases.

Answers: 2
Another question on Physics

Physics, 22.06.2019 02:30
Awave travels at a speed of 5.2 m/s. if the distance between crests is 0.40 m, what is the frequency of the wave? use f=v/wavelength a. 13 hz b. 2.1 hz c. 0.077 hz d. 5.6 hz
Answers: 1

Physics, 22.06.2019 02:30
The act of moving from one place to another. a. locomotor b. aerobic c. stationary d. static
Answers: 3


Physics, 22.06.2019 14:30
Suppose that 27 j of work is needed to stretch a spring from its natural length of 6 m to a length of 9 m. (a) how much work is needed to stretch the spring from 12 m to 14 m? j (b) how far beyond its natural length will a force of 78 n keep the spring stretched?
Answers: 2
You know the right answer?
The latent heat of vaporization for water at room temperature is 2430 J/g.
1. How much kinetic ener...
Questions


Geography, 11.10.2020 22:01

History, 11.10.2020 22:01


Mathematics, 11.10.2020 22:01


Computers and Technology, 11.10.2020 22:01

Social Studies, 11.10.2020 22:01


Mathematics, 11.10.2020 22:01

Mathematics, 11.10.2020 22:01

English, 11.10.2020 22:01


History, 11.10.2020 22:01

Biology, 11.10.2020 22:01

Physics, 11.10.2020 22:01



English, 11.10.2020 22:01

Advanced Placement (AP), 11.10.2020 22:01