
Physics, 12.01.2021 08:40 mmvill0809
A 300.0 g sample of water at 80.0°C is mixed with 300.0 g of water at
10.0°C. Assuming no heat loss to the surroundings, what is the final
temperature of the mixture? The specific heat capacity of liquid water is
4180 J/kg.°C *
Will give brainliest and lot of stars please help

Answers: 2
Another question on Physics

Physics, 22.06.2019 00:50
Part a constants huck finn walks at a speed of 0.60 m/s across his raft (that is, he walks perpendicular to the raft's motion what is huck's velocity (speed and direction) relative to the river bank? express your answer to three significant figures and include the appropriate units. relative to the shore)
Answers: 3

Physics, 22.06.2019 10:00
If a stone with an original velocity of 0 is falling from a ledgeand takes 8 seconds to hoybthe ground whays the final velocity of the stone
Answers: 2


Physics, 22.06.2019 13:30
6–48 bananas are to be cooled from 24 to 138c at a rate of 215 kg/h by a refrigeration system. the power input to the refrigerator is 1.4 kw. determine the rate of cooling, in kj/ min, and the cop of the refrigerator. the specific heat of banana above freezing is 3.35 kj/kg·8c.
Answers: 3
You know the right answer?
A 300.0 g sample of water at 80.0°C is mixed with 300.0 g of water at
10.0°C. Assuming no heat loss...
Questions

Mathematics, 10.02.2021 20:20

English, 10.02.2021 20:20


English, 10.02.2021 20:20


History, 10.02.2021 20:20


Mathematics, 10.02.2021 20:20






Mathematics, 10.02.2021 20:20

Mathematics, 10.02.2021 20:20

Mathematics, 10.02.2021 20:20


Chemistry, 10.02.2021 20:20

Arts, 10.02.2021 20:20

Mathematics, 10.02.2021 20:20

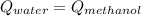
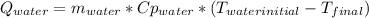
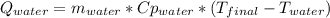
![0.3*4180*(80-T_{final})=0.3*4180*(T_{final}-10)\\100320-1254*T_{final}=1254*T_{final}-12540\\112860=2508*T_{final}\\T_{final}=45[C]](/tpl/images/1028/6130/52afa.png)


