

Answers: 3
Another question on Physics

Physics, 22.06.2019 15:30
Ametal ring 4.20 cm in diameter is placed between the north and south poles of large magnets with the plane of its area perpendicular to the magnetic field. these magnets produce an initial uniform field of 1.12 t between them but are gradually pulled apart, causing this field to remain uniform but decrease steadily at 0.240 t/s . (a) what is the magnitude of the electric field induced in the ring? (b) in which direction (clockwise or counterclockwise) does the current flow as viewed by someone on the south pole of the magnet?
Answers: 2

Physics, 22.06.2019 15:30
Two pans of a balance are 24.1 cm apart. the fulcrum of the balance has been shifted 1.33 cm away from the center by a dishonest shopkeeper. by what percentage is the true weight of the goods being marked up by the shopkeeper? assume the balance has negligible mass. answer in units of %.
Answers: 1

Physics, 22.06.2019 16:30
When measuring volume, why is it important to use the correct tools and units to attain the greatest accuracy? use specific examples from the lab to support your answer. (5 points)
Answers: 1

Physics, 22.06.2019 19:20
Two kilograms of air within a piston–cylinder assembly executes a carnot power cycle with maximum and minimum temperatures of 800 k and 300 k, respectively. the heat transfer to the air during the isothermal expansion is 60 kj. at the end of the isothermal expansion the volume is 0.4 m3. assume the ideal gas model for the air. determine the thermal efficiency, the volume at the beginning of the isothermal expansion, in m3, and the work during the adiabatic expansion, in kj.
Answers: 1
You know the right answer?
KI + Cl2 ---> KCl + I2
Balance the single replacement chemical reaction....
Balance the single replacement chemical reaction....
Questions


Geography, 03.02.2020 15:02


Mathematics, 03.02.2020 15:02



English, 03.02.2020 15:02


Geography, 03.02.2020 15:02

Health, 03.02.2020 15:02



Biology, 03.02.2020 15:02

English, 03.02.2020 15:02

Mathematics, 03.02.2020 15:02

Social Studies, 03.02.2020 15:02

Mathematics, 03.02.2020 15:02



Mathematics, 03.02.2020 15:02

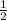 , b =
, b = 

