
Physics, 29.01.2021 06:40 gustavoroggero39
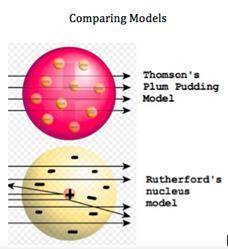
Atomic models have changed over the decades. Two early atomic models can be seen here. There is a dramatic change in the models, as Rutherford experimented with the cathode ray tube and charged particles. Differentiate between the two models by selecting the main difference between the models.
Question 5 options:
Rutherford's model shows negative charges dispersed throughout the atom
Rutherford's model shows negative particles orbiting the central nucleus
Rutherford's model shows the positive charge of an atom as a very small area
Thomson's model shows at sea of negative charged particles surrounding a small, positive area

Answers: 2
Another question on Physics

Physics, 21.06.2019 18:00
Acommercial passenger jet can travel up to 970 km/hr at an altitude of 30,000 feet. at this speed, how long will it take the jet to travel 1,865 kilometers? a. 2.3 hrs b. 1.9 hrs c. 1.7 hrs d. 1.5 hrs
Answers: 1


Physics, 22.06.2019 08:00
A0.580-kg rock is tied to the end of a string and is swung in a circle with a radius of 0.500 meters. the velocity of the rock is 4.50 m/s. what is the centripetal force acting on the rock? 15.5 n 5.22 n 69.8 n 23.5 n
Answers: 2

Physics, 22.06.2019 08:00
Which graph represents motion with an object with positive velocity that is located at a position of 3 meters at a time of 0 seconds? a) a b) bb eliminate c) c d) d
Answers: 1
You know the right answer?
Atomic models have changed over the decades. Two early atomic models can be seen here. There is a dr...
Questions


History, 03.09.2020 21:01


English, 03.09.2020 21:01






Mathematics, 03.09.2020 21:01




English, 03.09.2020 21:01


Mathematics, 03.09.2020 21:01


Mathematics, 03.09.2020 21:01




