Hi.
I need help with this question :
A piece of copper of mass 40g at 200°C is placed in...

Physics, 01.02.2021 01:30 kharmaculpepper
Hi.
I need help with this question :
A piece of copper of mass 40g at 200°C is placed in a copper calorimeter of mass 60g containing 50g of water at 25°C. Ignoring heat losses, what will be the final temperature of the mixture.
(Specific heat capacity of copper= 0.4J/g/K, specific heat capacity of water= 4.2J/g/K )?
Please show workings.

Answers: 3
Another question on Physics

Physics, 22.06.2019 05:30
If gases like carbon dioxide and methane make up less than 1% of the total atmosphere, why is it important for scientists to monitor changes in percentages of these gases?
Answers: 1

Physics, 22.06.2019 11:00
What would be the result of an alpha particle coming into a magnetic field? a) the alpha particle will stop moving. b) the alpha particle will reverse its direction. c) the alpha particle will be deflected in a curve path. d) the alpha particle will continue to travel in a straight line.
Answers: 1

Physics, 22.06.2019 12:30
Which governments provide garbage collection services to homes and businesses
Answers: 3

Physics, 22.06.2019 14:40
Glass has a hardness that is in the middle of the hardness scale. what is the hardness of glass?
Answers: 1
You know the right answer?
Questions

English, 28.09.2021 18:30



Geography, 28.09.2021 18:30

Mathematics, 28.09.2021 18:30




Mathematics, 28.09.2021 18:30


Mathematics, 28.09.2021 18:30

Computers and Technology, 28.09.2021 18:30



Mathematics, 28.09.2021 18:30


Mathematics, 28.09.2021 18:30



 specific heat capacity,
specific heat capacity,  initial temperature,
initial temperature, 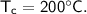
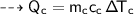
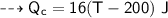
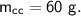 specific heat capacity,
specific heat capacity, 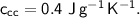 initial temperature,
initial temperature, 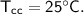
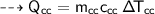
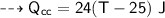
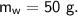 specific heat capacity,
specific heat capacity, 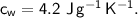 initial temperature,
initial temperature, 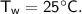
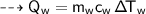
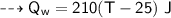
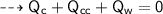
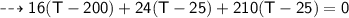

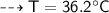

 to K or K to
to K or K to 


