Physics, 11.02.2021 20:30 MayFlowers
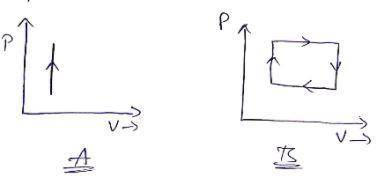
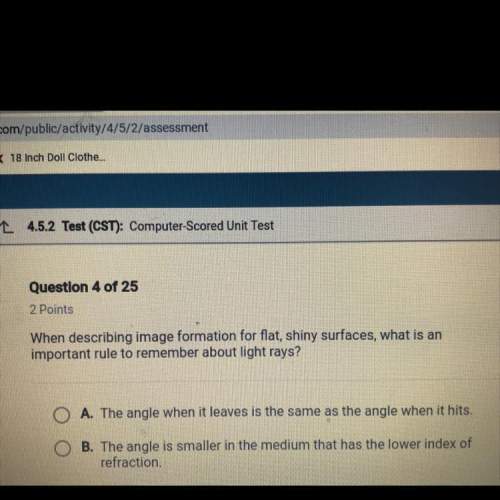
Two 800 cm^3 containers hold identical amounts of a monatomic gas at 20°C. Container A is rigid. Container B has a 100 cm^2 piston with a mass of 10 kg that can slide up and down vertically without friction. Both containers are placed on identical heaters and heated for equal amounts of time.
Required:
a. Will the final temperature of the gas in A be greater, less than, or equal to the temperature in B?
b. Show both processes on a single PV diagram.
c. What are the initial pressures in containers A and B?
d. Suppose the heaters have 25 W of power and are turned on for 15s. What is the final volume of container B?

Answers: 3
Another question on Physics


Physics, 22.06.2019 16:20
What is the single most important equation in all of physics?
Answers: 1

Physics, 23.06.2019 01:30
Aball is thrown vertically upwards from the top of a tower with a speed of 100m/s.it strikes the pound near the base of the tower after 25sec . the height of the tower is
Answers: 3

Physics, 23.06.2019 13:00
Why, on the molecular level, does changing the amount of baking soda or vinegar affect the amount of carbon dioxide gas produced?
Answers: 2
You know the right answer?
Two 800 cm^3 containers hold identical amounts of a monatomic gas at 20°C. Container A is rigid. Co...
Questions

Mathematics, 12.11.2020 23:10


Biology, 12.11.2020 23:10

Social Studies, 12.11.2020 23:10

Mathematics, 12.11.2020 23:10

English, 12.11.2020 23:10

Mathematics, 12.11.2020 23:10



Arts, 12.11.2020 23:10

Health, 12.11.2020 23:10





Mathematics, 12.11.2020 23:10

World Languages, 12.11.2020 23:10

Mathematics, 12.11.2020 23:10

French, 12.11.2020 23:10

Mathematics, 12.11.2020 23:10