
Physics, 11.02.2021 23:30 pennygillbert
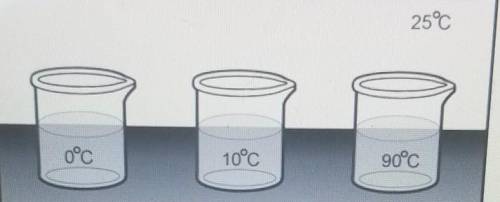
Three beakers containing the same volume of water at three different temperatures are placed in a room with air at standard air temperature, Which of the following will be the most likely result?
The amount of total energy in the water molecules for all three beakers will increase
The temperature in all three beakers will eventually be 25c.
The beaker at Oc will increase to 90c
The surrounding room temperature will eventually be 100c

Answers: 1
Another question on Physics

Physics, 22.06.2019 07:30
Some material consisting of a collection of microscopic objects is kept at a high temperature. a photon detector capable of detecting photon energies from infrared through ultraviolet observes photons emitted with energies of 0.3 ev, 0.5 ev, 0.8 ev, 2.0ev, 2.5ev, and 2.8ev. these are the only photon energies observed. (a) draw and label a possible energy-level diagram for one of the microscopic objects, which has four bound states. on the diagram, indicate the transitions corresponding to the emitted photons. explain briefly. (b) would a spring–mass model be a good model for these microscopic objects? why or why not? (c) the material is now cooled down to a very low temperature, and the photon detector stops detecting photon emissions. next, a beam of light with a continuous range of energies from infrared through ultraviolet shines on the material, and the photon detector observes the beam of light after it passes through the material. what photon energies in this beam of light are observed to be significantly reduced in intensity (“dark absorption lines”)? explain briefly.
Answers: 3

Physics, 22.06.2019 07:50
Calculate the ratio of h+ ions to oh– ions at a ph = 6. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 6. are they the same? why or why not? record your explanation in table a. what is the concentration of h+ ions at a ph = 6? mol/l what is the concentration of oh– ions at a ph = 6? mol/l what is the ratio of h+ ions to oh– ions at a ph = 6? : 1
Answers: 1

Physics, 22.06.2019 11:30
If forces acting on an object are unbalanced. true or false
Answers: 1

Physics, 22.06.2019 11:30
Why is the energy that results from a roller coaster's position at the top of a hill referred to as potential energy?
Answers: 1
You know the right answer?
Three beakers containing the same volume of water at three different temperatures are placed in a ro...
Questions




Mathematics, 17.12.2020 01:20


Physics, 17.12.2020 01:20


Mathematics, 17.12.2020 01:20


Mathematics, 17.12.2020 01:20





Mathematics, 17.12.2020 01:20

Mathematics, 17.12.2020 01:20

Physics, 17.12.2020 01:20

Chemistry, 17.12.2020 01:20





