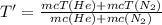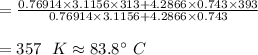PROBLEM 5 (Problem 4-145 in 7th edition) Consider a well-insulated horizontal rigid cylinder that is divided into two compartments by a piston that is free to move but does not allow either gas to leak into the other side. Initially, one side of the piston contains 1 m3 of N2 gas at 500 kPa and 120oC while the other side contains 1 m3 of He gas at 500 kPa and 40oC. Assume the piston is made of 8 kg of copper initially at the average temperature of the two gases on both sides. Now thermal equilibrium is established in the cylinder as a result of heat transfer through the piston. Using constant specific heats at room temperature, determine the final equilibrium temperature in the cylinder. What would your answer be if the piston were not free to move

Answers: 3
Another question on Physics

Physics, 21.06.2019 19:30
Astone dropped into a well is heard to strike the water after 8seconds. find the depth of the well if the velocity of sound is 350m/s
Answers: 1

Physics, 22.06.2019 04:00
Determine the maximum r-value of the polar equation r =3+3 cos 0
Answers: 3

Physics, 22.06.2019 07:30
Carbon-14 is a radioactive element that undergoes beta decay. which force is responsible for allowing carbon-14 to become stable? electromagnetic gravitational weak nuclear strong nuclear
Answers: 2

Physics, 22.06.2019 09:00
When a light bulb shines, it gives off light energy and energy. a. heat b. potential c. chemical d. electrical
Answers: 2
You know the right answer?
PROBLEM 5 (Problem 4-145 in 7th edition) Consider a well-insulated horizontal rigid cylinder that is...
Questions





Mathematics, 05.03.2020 13:04

Biology, 05.03.2020 13:04





Geography, 05.03.2020 13:05




Mathematics, 05.03.2020 13:06




Mathematics, 05.03.2020 13:07

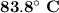 ".
".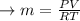

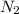 :
: