
Physics, 03.03.2021 21:30 JusSomeRandomGuy
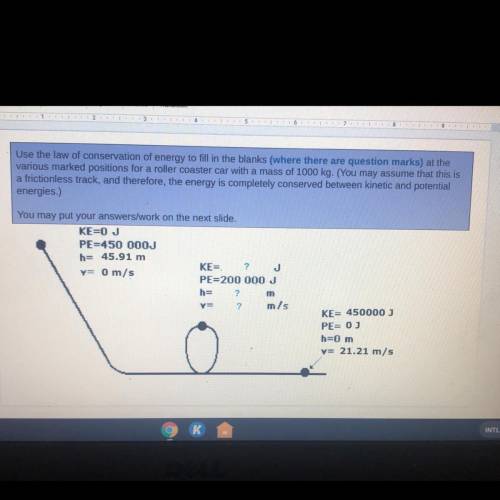
Use the law of conservation of energy to fill in the blanks (where there are question marks) at the
various marked positions for a roller coaster car with a mass of 1000 kg. (You may assume that this is
a frictionless track, and therefore, the energy is completely conserved between kinetic and potential
energies.)

Answers: 1
Another question on Physics

Physics, 22.06.2019 08:30
Abike rider starts from rest and accelerates 28.0 meters down a slope in 5.00 seconds. what is her acceleration? select one: a. 3.21 m/sec2 b. 1.75 m/sec2 c. 9.80 m/sec2 d. 2.24 m/sec2
Answers: 3

Physics, 22.06.2019 19:30
How many electrons in an atom could have these sets of quantum numbers? =3n=3 electronselectrons =4,ℓ=2n=4,ℓ=2 electronselectrons =6,ℓ=ℓ=−1n=6,ℓ=2,mℓ=−1
Answers: 3

Physics, 22.06.2019 21:50
Which of the following is part of the explanation or description of electromagnetic waves? (select all that apply) a. electrically charged molecules in a medium transfer their energy to nearby electrically charged molecules. b. a changing electric field causes a changing magnetic field. c. magnetic molecules in a medium transfer their energy to nearby magnetic molecules. d. a changing magnetic field causes a changing electric field.
Answers: 3

Physics, 23.06.2019 09:30
How many milliliters of water at 23 °c with a density of 1.00 g/ml must be mixed with 180 ml (about 6 oz) of coffee at 95 °c so that the resulting combination will have a temperature of 60 °c? assume that coffee and water have the same density and the same specific heat. how much will the temperature of a cup (180 g) of coffee at 95 °c be reduced when a 45 g silver spoon (specific heat 0.24 j/g °c) at 25 °c is placed in the coffee and the two are allowed to reach the same temperature? assume that the coffee has the same density and specific heat as water. a 45-g aluminum spoon (specific heat 0.88 j/g °c) at 24 °c is placed in 180 ml (180 g) of coffee at 85 °c and the temperature of the two become equal. (a) what is the final temperature when the two become equal? assume that coffee has the same specific heat as water. (b) the first time a student solved this problem she got an answer of 88 °c. explain why this is clearly an incorrect answer.
Answers: 1
You know the right answer?
Use the law of conservation of energy to fill in the blanks (where there are question marks) at the...
Questions


Chemistry, 23.07.2019 12:30

Mathematics, 23.07.2019 12:30


Geography, 23.07.2019 12:30


Social Studies, 23.07.2019 12:30




History, 23.07.2019 12:30


Mathematics, 23.07.2019 12:30






Health, 23.07.2019 12:30



