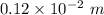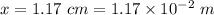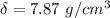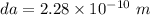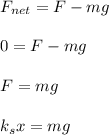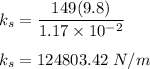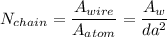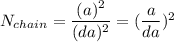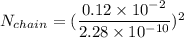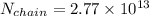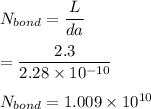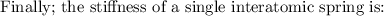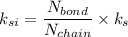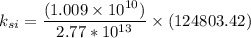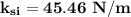Physics, 06.03.2021 01:00 denisebaslee15
One mole of iron (6 x 10^23 atoms) has a mass of 56 grams, and its density is 7.87 grams per cubic centimeter, so the center-to-center distance between atoms is 2.28 x 10^-10 m. You have a long thin bar of iron, 2.3 m long, with a square cross section, 0.12 cm on a side. You hang the rod vertically and attach a 149 kg mass to the bottom, and you observe that the bar becomes 1.17 cm longer. From these measurements, it is possible to determine the stiffness of one interatomic bond in iron.
ks = 20014.1 N/m
Number of side-by-side long chains of atoms = 4.81e^12
Number of bonds in total length = 1.096e^10
What is the stiffness of a single interatomic "spring"?

Answers: 1
Another question on Physics


Physics, 22.06.2019 11:30
A100-watt light bulb illuminates a solar cell. the electricity from the solar cell operates a water pump that delivers 1 watt of power. what is the efficiency of the system?
Answers: 2

Physics, 23.06.2019 01:00
Are formed where bumps from two surfaces come into contact ?
Answers: 1

Physics, 23.06.2019 02:00
Which of the following are molecules? a. al b. ag c. mgcl2 d. nacl e. c3h8
Answers: 1
You know the right answer?
One mole of iron (6 x 10^23 atoms) has a mass of 56 grams, and its density is 7.87 grams per cubic c...
Questions

Mathematics, 27.02.2021 02:30

Mathematics, 27.02.2021 02:30


Mathematics, 27.02.2021 02:30

Mathematics, 27.02.2021 02:30



English, 27.02.2021 02:30

Mathematics, 27.02.2021 02:30

Social Studies, 27.02.2021 02:30


Mathematics, 27.02.2021 02:30


English, 27.02.2021 02:30

Mathematics, 27.02.2021 02:30


German, 27.02.2021 02:30

Mathematics, 27.02.2021 02:30