
A frictionless piston-cylinder contains carbon dioxide gas (CO2) initially at 500oC and 2 MPa. The system is o3 cooled in an isobaric process until the final temperature becomes 350 C and the final volume is 1 m . For this process, determine: (a) The reduced pressure and the reduced temperature of the initial state. (b) The initial volume of the piston-cylinder (in m3). (c) The mass of CO2 in the piston-cylinder (in kg). (d) The total boundary work for the process (in kJ). (e) The amount of heat transfer during the cooling process (in kJ).

Answers: 2
Another question on Physics

Physics, 21.06.2019 14:00
Consider a resistor with color code “orange blue brown gold” and a measured value of 370 ω. determine the following: nominal value, tolerance, % error, and the probability of another resistor from the same manufacturing batch having a value closer to nominal than this one. use matlab or excel to evaluate the erf function.
Answers: 2


Physics, 22.06.2019 19:30
What characteristics of hv2112 make it the best candidate to be classified as a thorne-zytkow object?
Answers: 3

Physics, 23.06.2019 01:30
Aplane's average speed between two cities is 600 km/h. if the trip takes 2.5 hours, how far does the plane fly?
Answers: 2
You know the right answer?
A frictionless piston-cylinder contains carbon dioxide gas (CO2) initially at 500oC and 2 MPa. The s...
Questions

Business, 06.05.2020 08:03

Mathematics, 06.05.2020 08:03


Mathematics, 06.05.2020 08:03

Mathematics, 06.05.2020 08:03


Physics, 06.05.2020 08:03

Biology, 06.05.2020 08:03



Chemistry, 06.05.2020 08:03

Mathematics, 06.05.2020 08:03

Mathematics, 06.05.2020 08:03


History, 06.05.2020 08:03



Physics, 06.05.2020 08:03


Biology, 06.05.2020 08:03

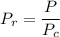
 = 7.3773 MPa
= 7.3773 MPa
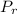 = 0.2711 MPa
= 0.2711 MPa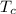 = 304.13 K
= 304.13 K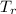 , is given by the following formula;
, is given by the following formula;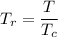
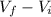 )
)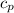 ×(T₂ - T₁)
×(T₂ - T₁)

