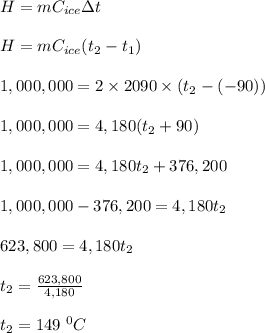The specific heat of water in its solid phase (ice) is 2090 J/(kg K), while in the liquid phase (water) its specific heat is 4190 J/(kg K). Water's latent heat of fusion is 333,000 J/kg. If you have a 2kg block of ice at -90°C and you add 1,000,000 J of heat, what is its new temperature?
a. 0°C
b. 14°C
c. 49°C
d. 149°C

Answers: 1
Another question on Physics

Physics, 21.06.2019 22:30
Ann walks 80 meters on a straight line 33 ∘ north of east starting at point 1. draw ann's path. represent ann's walk with a vector of length 80 meters. draw the vector starting at point 1. the length given in the display is in meters.
Answers: 1

Physics, 22.06.2019 00:30
Two vectors are shown. which statement best compares the vectors? vector x has greater magnitude than vector y. vector y has greater magnitude than vector x. the vectors have the same displacement. the vectors have the same direction.
Answers: 1

Physics, 22.06.2019 05:00
Which statements describe the movement of ocean currents around the globe? check all that apply. strong winds force warm water to sink to the ocean floor. the coriolis effect causes warm and cold water to mix. cool dense water sinks to the ocean floor. warm water replaces cool surface water. wind blowing parallel to the shore causes upwelling of cool water.
Answers: 1

Physics, 22.06.2019 05:40
The difference between a red shift and a blue shift has to do with wavelength frequency. t or f
Answers: 1
You know the right answer?
The specific heat of water in its solid phase (ice) is 2090 J/(kg K), while in the liquid phase (wat...
Questions


History, 11.02.2020 23:29


Mathematics, 11.02.2020 23:29


Biology, 11.02.2020 23:29

Mathematics, 11.02.2020 23:29




Mathematics, 11.02.2020 23:29








Health, 11.02.2020 23:30

Mathematics, 11.02.2020 23:30