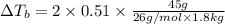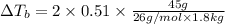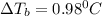Amixture of two gases exerts a total pressure of 3.65 atm. if the partial pressure of one of the gases is 1.86 atm, what is the partial pressure of the other gas in the mixture?
answer
0.510 atm
1.79 atm
1.96 atm
6.79 atm
if 45 g of lif are dissolved in 1.8 kg of water, what would be the expected change in boiling point? the boiling point constant for water (kb) is 0.51 °c/m.
answer
0.49°c
0.98°c
1.9°c
3.5°c

Answers: 3
Another question on Physics


Physics, 22.06.2019 09:30
The necleus of an atom is made up of what subatomic particles?
Answers: 1

Physics, 22.06.2019 10:00
Need people build a dam to create a reservoir that supplies water a nearby city needs. describe two ways this action will likely affect the water cycle in the local environment. (5 points) worth 20 points
Answers: 1

Physics, 22.06.2019 16:30
One number is said to be an "order of magnitude" larger than another number if choose one: a. it is 10 times larger. b. it is 5 times larger. c. it is 3 times larger. d. it is 100 times larger. e. it is 2 times larger.
Answers: 1
You know the right answer?
Amixture of two gases exerts a total pressure of 3.65 atm. if the partial pressure of one of the gas...
Questions

English, 27.01.2021 19:00

Mathematics, 27.01.2021 19:00

Mathematics, 27.01.2021 19:00

Chemistry, 27.01.2021 19:00


Mathematics, 27.01.2021 19:00

Mathematics, 27.01.2021 19:00

Mathematics, 27.01.2021 19:00

Physics, 27.01.2021 19:00

Mathematics, 27.01.2021 19:00


Mathematics, 27.01.2021 19:00

Mathematics, 27.01.2021 19:00

Mathematics, 27.01.2021 19:00


Mathematics, 27.01.2021 19:00



Mathematics, 27.01.2021 19:00

Mathematics, 27.01.2021 19:00

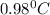
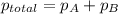
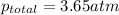
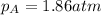
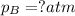
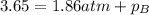
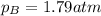
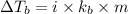
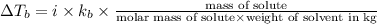
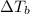 = elevation in boiling point
= elevation in boiling point 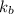 = boiling point constant
= boiling point constant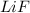 , i= 2 as it dissociates to give two ions.
, i= 2 as it dissociates to give two ions.