
Physics, 02.10.2019 17:40 zhenhe3423
The pressure exerted on a sample of a fixed amount of gas is doubled at constant temperature, and then the temperature of the gas in kelvins is doubled at constant pressure.
what is the final volume of the gas?
what is the final volume of the gas?
the final volume is twice the initial volume.
the final volume of the gas is one-fourth the initial volume.
the final volume of the gas is the same as the initial volume.
the final volume of the gas is four times the initial volume.
the final volume of the gas is one-half the initial volume.

Answers: 1
Another question on Physics


Physics, 22.06.2019 02:40
If the wheels lock when braking suddenly the vehicle: a: loses traction b: lose steering wheel ability c: gain speed slightly d: gain steering ability
Answers: 1

Physics, 22.06.2019 04:30
Afeather of mass 0.001 kg falls from a height of 2 m. under realistic conditions, it experiences air resistance. based on what you know about friction, what can you say about the kinetic energy of the feather as it reaches the ground? acceleration due to gravity is g = 9.8 m/s2. a. ke < 0.0196 j b. ke = 00196 j c. je = 0 j d. ke > 0.0196 j
Answers: 1

Physics, 22.06.2019 06:20
Part 1: a magnetic levitation or maglev train rides rails without touching them. explain how this works using your data. include the appropriate magnet drawing in your answer. part 2: two objects are near a bar magnet. one is about 1 cm away, while the other is 6 cm away. compare and contrast the magnetic force that affects each object. use your data to answer the question
Answers: 1
You know the right answer?
The pressure exerted on a sample of a fixed amount of gas is doubled at constant temperature, and th...
Questions




Mathematics, 10.11.2020 21:20

Mathematics, 10.11.2020 21:20

Physics, 10.11.2020 21:20







Mathematics, 10.11.2020 21:20

Mathematics, 10.11.2020 21:20

Mathematics, 10.11.2020 21:20




Law, 10.11.2020 21:20

 (at constant temperature and number of moles)
(at constant temperature and number of moles)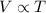 (at constant pressure and number of moles)
(at constant pressure and number of moles)

