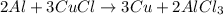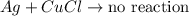Physics, 18.01.2020 18:31 isabelacarraler
In an experiment the chemical reaction between a piece of aluminum foil and copper(ii)chloride solution in a beaker is observed. the aluminum seems to disapear over time. the balanced chemical equation is given by: 2 al + 3 cucl ⇒3 cu +2 alcl3
can you predict the outcome of the same experiment when aluminum is replaced with a more reactive element such as silver?
a) no reaction would occur.
b) the silver would disappear but no solid precipitate would form.
c) the silver would disappear and a silver solid precipitate would form.
d) the silver would disappear and a brownish red solid precipitate would form.
hint: what would the new product be?

Answers: 1
Another question on Physics

Physics, 22.06.2019 05:30
Which of the choices below is one of the primary gases found in the atmosphere? a. helium b. carbon dioxide c. nitrogen d. argon
Answers: 2

Physics, 22.06.2019 06:20
Three charge are arranged as shown in the diagram. the magnitude of the net electrical force acting on the +6 uc charge, rounded to the tenths place, is .
Answers: 1

Physics, 22.06.2019 14:30
Which compound is held together by the electrostatic force between two ions? a. co2 b. cci4 c. h2s d. mgf2
Answers: 1

Physics, 23.06.2019 05:00
Heating water for hot cocoa while sitting next to a campfire can be examples of conduction, convenction and radiation at the same time agree disagree it depends how you know
Answers: 2
You know the right answer?
In an experiment the chemical reaction between a piece of aluminum foil and copper(ii)chloride solut...
Questions






English, 28.06.2019 20:30

Biology, 28.06.2019 20:30


Biology, 28.06.2019 20:30

Social Studies, 28.06.2019 20:30

Biology, 28.06.2019 20:30


Biology, 28.06.2019 20:30


Mathematics, 28.06.2019 20:30

Mathematics, 28.06.2019 20:30



Mathematics, 28.06.2019 20:30

Arts, 28.06.2019 20:30