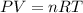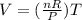Physics, 29.01.2020 10:54 lucyfox8165
Ascientist tested charles’s law, which states that volume is directly related to temperature. assume constant pressure. what will the scientist observe?
a) as volume decreases, temperature increases
b) as volume decreases, temperature decreases
c) as volume increases, temperature stays constant
d) as volume decreases, temperature can increase or decrease

Answers: 1
Another question on Physics

Physics, 21.06.2019 22:30
Select the word from the list that best fits the definition classification of most stars on the h-r diagram
Answers: 1

Physics, 21.06.2019 23:30
Which lists the main components of darwin’s theory of evolution? a. random mutations drive evolution; the evolution of a population happens slowly; organisms have common ancestors; organisms do not change. b. natural selection drives evolution; the evolution of a population happens slowly; organisms have common ancestors; organisms change over time. c. natural selection drives evolution; the evolution of a population happens rapidly; organisms have common ancestors; organisms change over time. d. random mutations drive evolution; the evolution of a population happens rapidly; organisms have common ancestors; organisms do not change.
Answers: 1

Physics, 22.06.2019 00:00
What must be the same for any two resistors that are connected in series ? a. the potntial diffrence across each b. the rate at which charge moves through each c. the rate at which energy is used by each d. the resistance to the flow of charge of each
Answers: 1

Physics, 22.06.2019 00:10
The energy released by a chemical reaction can be measured using a calorimeter. when barium hydroxide octahydrate crystals are reacted with dry ammonium chloride inside of a coffee cup calorimeter, the temperature of the 18.00 g of water in the calorimeter decreases from 30.0°c to 8.0°c. the equation for calculating energy absorbed or released by a reaction is: where q is the energy released or absorbed, m is the mass of water in the calorimeter, cp is the specific heat of water, and δt is the observed temperature change. if the specific heat of liquid water is 4.19 j/g·°c, how much energy was absorbed by the reaction?
Answers: 3
You know the right answer?
Ascientist tested charles’s law, which states that volume is directly related to temperature. assume...
Questions

Mathematics, 04.10.2020 14:01

Mathematics, 04.10.2020 14:01



English, 04.10.2020 14:01





English, 04.10.2020 14:01

Arts, 04.10.2020 14:01


English, 04.10.2020 14:01




Mathematics, 04.10.2020 14:01



English, 04.10.2020 14:01