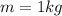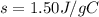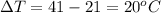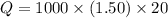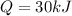Physics, 27.01.2020 19:31 khalid7746
Asample of octane (c8h18) that has a mass of 0.750 g is burned in a bomb calorimeter. as a result, the temperature of the calorimeter increases from 21.0°c to 41.0°c. the specific heat of the calorimeter is 1.50 j/(g • °c), and its mass is 1.00 kg. how much heat is released during the combustion of this sample? use mc021-1.jpg. 22.5 kj 30.0 kj 31.5 kj 61.5 kj

Answers: 2
Another question on Physics

Physics, 21.06.2019 22:00
Na food processing facility, a spherical container of inner radius r1 5 40 cm, outer radius r2 5 41 cm, and ther- mal conductivity k 5 1.5 w/m · k is used to store hot water and to keep it at 100°c at all times. to accomplish this, the outer surface of the container is wrapped with a 800-w electric strip heater and then insulated. the temperature of the inner surface of the container is observed to be nearly 120°c at all times. as- suming 10 percent of the heat generated in the heater is lost through the insulation, (a) express the differential equation and the boundary conditions for steady one-dimensional heat conduction through the container, (b) obtain a relation for the variation of temperature in the container material by solving the differential equation, and (c) evaluate the outer surface tempera- ture of the container. also determine how much water at 100°c this tank can supply steadily if the cold water enters at 20°c.
Answers: 2

Physics, 21.06.2019 22:30
The percent efficiency of a machine can never be 100% (or greater), because in the real world some energy is always converted into a. heat b. work c. input force d. output force
Answers: 1

Physics, 21.06.2019 22:30
The of a machine determines its "usefulness." a. input force b. output force c. mechanical advantage
Answers: 2

You know the right answer?
Asample of octane (c8h18) that has a mass of 0.750 g is burned in a bomb calorimeter. as a result, t...
Questions

History, 04.08.2019 14:00


History, 04.08.2019 14:00

History, 04.08.2019 14:00





Mathematics, 04.08.2019 14:00





History, 04.08.2019 14:00




Geography, 04.08.2019 14:00