
Physics, 30.04.2021 01:40 bercishicicorbin
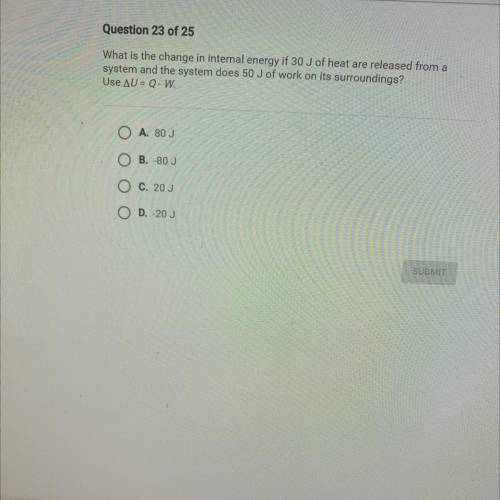
What is the change in internal energy if 30 J of heat are released from a
system and the system does 50 J of work on its surroundings?
Use AU= Q-W.

Answers: 1
Another question on Physics

Physics, 21.06.2019 15:00
Air flows upward in the wick of a lantern because of the liquid property called
Answers: 1

Physics, 21.06.2019 23:20
From center station, a train departs every 30 minutes on the fast line and a train departs every 50 minutes on the state line. if two trains depart from center station at 8: 00 a.m., one on each of the two lines, what is the next time that two trains, one on each line, will depart at the same time?
Answers: 1

Physics, 22.06.2019 10:00
There are two-speed cameras, one in an are where the speed limit is 30 km/h and another where the speed limit is 50 km/h. in which will there be a smaller time interval between the photographs? explain your answer.
Answers: 1

Physics, 23.06.2019 09:00
To find the mass defect for uranium-238 several calculations must be made. which is the calculation that should be made first? assume atomic number for uranium-238 is 92. a. 92(1.0073) x 92(1.0087) = b. 92(1.0073) x 146(1.0087) = c. 146(1.0073) x 238(1.0087) = d. 238(1.0073) x 238(1.0087) =
Answers: 1
You know the right answer?
What is the change in internal energy if 30 J of heat are released from a
system and the system do...
Questions



Geography, 15.04.2020 23:30

Mathematics, 15.04.2020 23:30




English, 15.04.2020 23:31

Advanced Placement (AP), 15.04.2020 23:31



Mathematics, 15.04.2020 23:31


Computers and Technology, 15.04.2020 23:31

Computers and Technology, 15.04.2020 23:31







