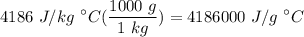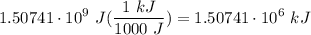Physics, 12.05.2021 01:20 Smitheyyy973
Q=mcAt Calculate the amount of heat gained by 114.32 grams of water at 14.85C raised to 18.0C. The heat capacity of water is 4186J/kgC.

Answers: 2
Another question on Physics

Physics, 21.06.2019 20:30
3. on your graph, the data points between the black squares are data for elements with atomic numbers 3 through 9. locate these elements on your periodic table. what term or description would you use to identify these elements with respect to the periodic table?
Answers: 2

Physics, 22.06.2019 00:20
Consider the particle-in-a-box problem in 1d. a particle with mass m is confined to move freely between two hard walls situated at x = 0 and x = l. the potential energy function is given as (a) describe the boundary conditions that must be satisfied by the wavefunctions ψ(x) (such as energy eigenfunctions). (b) solve the schr¨odinger’s equation and by using the boundary conditions of part (a) find all energy eigenfunctions, ψn(x), and the corresponding energies, en. (c) what are the allowed values of the quantum number n above? how did you decide on that? (d) what is the de broglie wavelength for the ground state? (e) sketch a plot of the lowest 3 levels’ wavefunctions (ψn(x) vs x). don’t forget to mark the positions of the walls on the graphs. (f) in a transition between the energy levels above, which transition produces the longest wavelength λ for the emitted photon? what is the corresponding wavele
Answers: 1


Physics, 22.06.2019 12:30
Aboy with a mass 25 kg climbs into a small tree. he sits on a branch that is 2.o m above to the ground.what is his gravitational potential energy above the ground?
Answers: 1
You know the right answer?
Q=mcAt
Calculate the amount of heat gained by 114.32 grams of water at 14.85C raised to 18.0C. The...
Questions








Mathematics, 05.03.2020 11:12



Computers and Technology, 05.03.2020 11:13







Mathematics, 05.03.2020 11:14