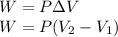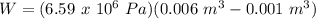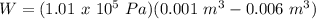A sample of gas, initially with a volume of 1.0 L, undergoes a thermodynamic cycle. Find the work done by the gas on its environment during each stage of the cycle described below. (Enter your answers in J.)
(a) First, the gas expands from a volume of 1.0 L to 6.0 L at a constant pressure of 6.5 atm.
(b) Second, the gas is cooled at constant volume until the pressure falls to 1.0 atm.
(c) Third, the gas is compressed at a constant pressure of 1.0 atm from a volume of 6.0 L to 1.0 L. (Note: Be careful of signs.)
(d) Finally, the gas is heated until its pressure increases from 1.0 atm to 6.5 atm at a constant volume. (e) What is the net work done by the gas on its environment during the complete cycle described above?

Answers: 1
Another question on Physics

Physics, 21.06.2019 20:10
Select the correct answer. anna will wear a black dress when she attends het cousin's wedding. which color(s) does the dress absorb? a. all colors b. no colors c. red and green d. white
Answers: 1


Physics, 22.06.2019 06:20
Part 1: a magnetic levitation or maglev train rides rails without touching them. explain how this works using your data. include the appropriate magnet drawing in your answer. part 2: two objects are near a bar magnet. one is about 1 cm away, while the other is 6 cm away. compare and contrast the magnetic force that affects each object. use your data to answer the question
Answers: 1

You know the right answer?
A sample of gas, initially with a volume of 1.0 L, undergoes a thermodynamic cycle. Find the work do...
Questions

Biology, 09.02.2021 22:10


Physics, 09.02.2021 22:10

Mathematics, 09.02.2021 22:10


Mathematics, 09.02.2021 22:10

Mathematics, 09.02.2021 22:10


Mathematics, 09.02.2021 22:10

Health, 09.02.2021 22:10

Mathematics, 09.02.2021 22:10


Mathematics, 09.02.2021 22:10

Mathematics, 09.02.2021 22:10





Mathematics, 09.02.2021 22:10