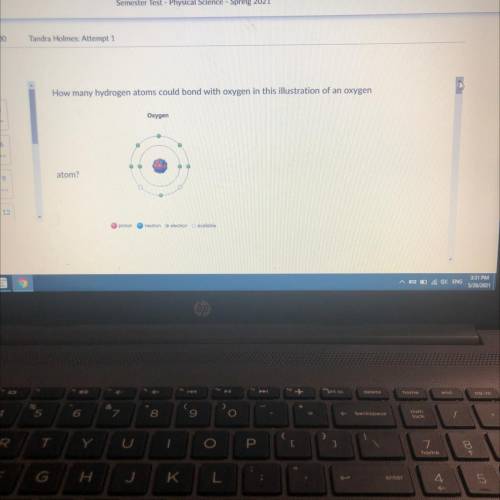
How many hydrogen atoms could bond with oxygen in this illustration of an oxygen
Oxygen
atom?...

Physics, 26.05.2021 23:50 samymaria1344
How many hydrogen atoms could bond with oxygen in this illustration of an oxygen
Oxygen
atom?
proton
neutron electron available
K12 D () ENG
5/

Answers: 1
Another question on Physics

Physics, 21.06.2019 16:30
Identify the amplitude on the diagram and give the correct description. question 3 options: a because amplitude is the distance from wave crest to wave crest. b because the wavelength shows amplitude. c because amplitude is the distance between a crest (or trough) and rest. d because wavelength show amplitude. (brainiest if you get it right! ) ~kayla
Answers: 1

Physics, 22.06.2019 05:30
Will give brainliest! which statement best describes the difference between strong nuclear forces and weak nuclear forces? weak nuclear forces are involved when certain types of atoms break down. strong nuclear forces are responsible for holding atoms' nucleus together. weak nuclear forces hold bonds between atoms together. strong nuclear forces hold together the nucleus of an atom. strong nuclear bonds prevent atoms from falling apart. weak nuclear bonds prevent compounds from falling apart. strong nuclear forces are involved in breaking electrons from their shells. weak nuclear forces hold protons in the nucleus.
Answers: 3

Physics, 22.06.2019 10:30
In science and physics what is the standard unit of measure for speed?
Answers: 1

Physics, 22.06.2019 12:00
Under the action of a constant force an object accelerates at 7.8 m/s2. what will the acceleration be if (a) the force is halved? (b) the object's mass is halved? (c) the force and the object's mass are both halved? (d) the force is halved and the object's mass is doubled?
Answers: 3
You know the right answer?
Questions

Mathematics, 23.09.2019 18:00

History, 23.09.2019 18:00



Mathematics, 23.09.2019 18:00

History, 23.09.2019 18:00

History, 23.09.2019 18:00

Biology, 23.09.2019 18:00


History, 23.09.2019 18:00

Law, 23.09.2019 18:00

Mathematics, 23.09.2019 18:00


History, 23.09.2019 18:00



English, 23.09.2019 18:00

English, 23.09.2019 18:00

Arts, 23.09.2019 18:00



