
Physics, 08.06.2021 01:00 gmoney1973
The liquid and gaseous state of hydrogen are in thermal equilibrium at 20.3 K. Even though it is on the point of condensation, model the gas as ideal and determine the most probable speed of the molecules (in m/s). What If? At what temperature (in K) would an atom of xenon in a canister of xenon gas have the same most probable speed as the hydrogen in thermal equilibrium at 20.3 K?

Answers: 1
Another question on Physics

Physics, 22.06.2019 13:40
Dao makes a table to identify the variables used in the equations for centripetal acceleration. what quantities belong in cells x and y?
Answers: 2

Physics, 22.06.2019 14:00
Select for each of the following statements whether it is correct or incorrect. (a) in an isothermal expansion of an ideal gas. (b) the temperature remains constant. (b) the pressure remains constant. (c) there is work done by the gas. (d) there is heat added to the gas. (e) the change in internal energy equals zero.
Answers: 1


Physics, 22.06.2019 22:00
Acar is traveling 35 mph on a smooth surface . if a balanced force is applied to the car what happens?
Answers: 3
You know the right answer?
The liquid and gaseous state of hydrogen are in thermal equilibrium at 20.3 K. Even though it is on...
Questions

Biology, 20.01.2021 01:00


Mathematics, 20.01.2021 01:00

Mathematics, 20.01.2021 01:00

Computers and Technology, 20.01.2021 01:00

Arts, 20.01.2021 01:00

Mathematics, 20.01.2021 01:00

Mathematics, 20.01.2021 01:00

Mathematics, 20.01.2021 01:00




Social Studies, 20.01.2021 01:00

Biology, 20.01.2021 01:00

Biology, 20.01.2021 01:00



Mathematics, 20.01.2021 01:00

English, 20.01.2021 01:00

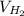 = √( 2RT /
= √( 2RT / 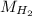 )
)

