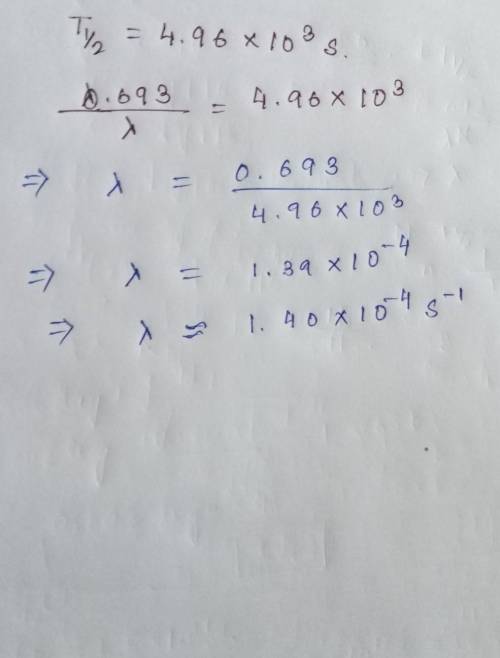Physics, 08.06.2021 08:50 twiddleturd
PLEASE HELP ME WITH THIS ONE QUESTION
The half-life of Barium-139 is 4.96 x 10^3 seconds. A sample contains 3.21 x 10^17 nuclei. What is the decay constant for this decay?
A) 1.67 x 10^-4 s^-1
B) 5.43 x 10^-4 s^-1
C) 1.40 x 10^-4 s^-1
D) 2.22 x 10^-4 s^-1

Answers: 3
Another question on Physics

Physics, 22.06.2019 05:50
High schoolphysics 5+3 pts a neon light consists of a glass tube with metal wires at each end. when connected to a high-voltage source, the gas glows. if a fly lands on the glass tube, what will most likely happen? a) the fly will not feel a shock because the glass conducts any free electrons back into the gas. b) electrons will flow directly from the metal wires along the glass and shock the fly. c) the fly will not feel a shock because the glass insulates it from the electrons in the gas and the metal. d) electrons that are moving through the gas will be conducted through the glass and shock the fly.
Answers: 3

Physics, 22.06.2019 08:30
Does anyone know how to solve this problem? i really need . i made an attempt but i just cant get it. a metal rod is 25.000 cm long at 25.0 degrees celsius. when heated to 102.0 degrees celsius, it is 25.054 cm long. what is the coefficient of linear expansion for this metal.
Answers: 3

Physics, 22.06.2019 10:00
(a) calculate the number of electrons in a small, electrically neutral silver pin that has a mass of 10.0 g. silver has 47 electrons per atom, and its molar mass is 107.87 g/mol. (b) imagine adding electrons to the pin until the negative charge has the very large value 1.00 mc. how many electrons are added for every 109 electrons already present
Answers: 3

Physics, 22.06.2019 15:50
Decreased sensitivity to an unchanging stimulus is known as
Answers: 3
You know the right answer?
PLEASE HELP ME WITH THIS ONE QUESTION
The half-life of Barium-139 is 4.96 x 10^3 seconds. A sample...
Questions




Biology, 17.10.2019 05:00




Mathematics, 17.10.2019 05:00



Social Studies, 17.10.2019 05:00

Biology, 17.10.2019 05:00


English, 17.10.2019 05:00


Mathematics, 17.10.2019 05:00

History, 17.10.2019 05:00

History, 17.10.2019 05:00