
Physics, 17.06.2021 15:10 montanolumpuy
If the outermost electron in an atom is excited to a very high energy state, its orbit is far beyond that of the other electrons. To a good approximation, we can think of the electron as orbiting a compact core with a charge equal to the charge of a single proton. The outer electron in such a Rydberg atom thus has energy levels corresponding to those of hydrogen.
Sodium is a common element for such studies. How does the radius you calculated in part A compare to the approximately 0.20 nm radius of a typical sodium atom?
r100/rNa = .

Answers: 2
Another question on Physics

Physics, 21.06.2019 14:30
How much work does the charge escalator do to move 2.30 μc of charge from the negative terminal to the positive terminal of a 3.00 v battery?
Answers: 1


Physics, 22.06.2019 05:30
Which of the following are considered noble gases? a. bromine b. neon c. argon d. chlorine
Answers: 1

Physics, 22.06.2019 09:00
An open cart is moving along a straight frictionless horizontal track. when rain starts falling vertically into the cart, what happens to the speed of the cart?
Answers: 3
You know the right answer?
If the outermost electron in an atom is excited to a very high energy state, its orbit is far beyond...
Questions

Biology, 25.03.2021 04:10



Mathematics, 25.03.2021 04:10

English, 25.03.2021 04:10


Chemistry, 25.03.2021 04:10

Mathematics, 25.03.2021 04:10




Mathematics, 25.03.2021 04:10


Biology, 25.03.2021 04:10

Mathematics, 25.03.2021 04:10

English, 25.03.2021 04:10

History, 25.03.2021 04:10

Mathematics, 25.03.2021 04:10


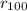 /
/ 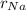 is 2645.0
is 2645.0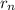 = n²α₀
= n²α₀

