
Physics, 18.06.2021 16:50 AnkitDavid1616
A 1-m3 tank holds a two-phase liquid-vapor mixture of carbon dioxide at – 17 °C. The quality of the mixture is 70%. For saturated carbon dioxide at – 17 °C, vf = 0.9827×10-3 m3/kg and vg =1.756×10-2 m3/kg. Determine the masses of saturated liquid and saturated vapor, each in kg. What is the percent of the total volume occupied by saturated liquid?

Answers: 2
Another question on Physics

Physics, 22.06.2019 08:00
5g of ammonium nitrate was dissolved in 60g of water in an insulated container. the temperature at the start of the reaction was 23.0°c and at the end it was 19.0°c. calculate the energy absorbed by the reaction.
Answers: 3

Physics, 22.06.2019 16:10
Amass weighing 24 pounds, attached to the end of a spring, stretches it 4 inches. initially, the mass is released from rest from a point 8 inches below the equilibrium position. find the equation of motion. (use g = 32 ft/s2 for the acceleration due to gravity.)
Answers: 1


You know the right answer?
A 1-m3 tank holds a two-phase liquid-vapor mixture of carbon dioxide at – 17 °C. The quality of the...
Questions

Mathematics, 25.05.2021 09:10

Mathematics, 25.05.2021 09:10



Business, 25.05.2021 09:10

Mathematics, 25.05.2021 09:10

History, 25.05.2021 09:10

Mathematics, 25.05.2021 09:10

Mathematics, 25.05.2021 09:10

Mathematics, 25.05.2021 09:10

Health, 25.05.2021 09:10

Chemistry, 25.05.2021 09:10

English, 25.05.2021 09:10

Mathematics, 25.05.2021 09:10

Mathematics, 25.05.2021 09:10




Mathematics, 25.05.2021 09:10

Mathematics, 25.05.2021 09:10

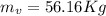
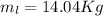
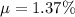
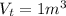
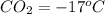
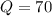
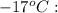

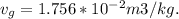
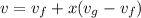
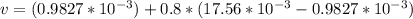
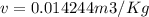
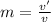
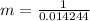
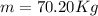

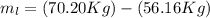
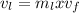
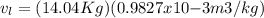
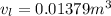
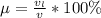
![\mu= [(0.01379 m^3)/(1 m^3)] *100 \%](/tpl/images/1378/7331/4b146.png)


