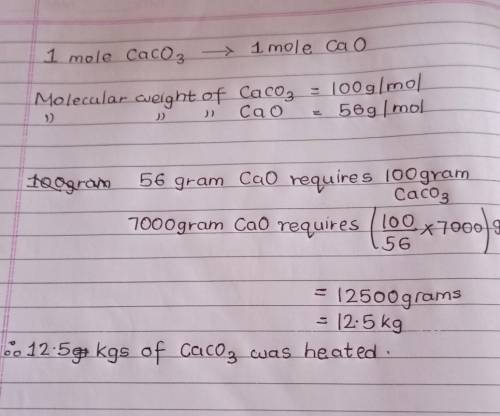Physics, 20.06.2021 14:00 mmunozgo9334
Quicklime which is calcium oxide, is made by heathig limestone in a furnace as per the equation :
CaCO3(s)CaO(s)+CO2
7.00Kg of calcium oxide was formed. what mass of calcium carbonate was heated?

Answers: 2
Another question on Physics

Physics, 22.06.2019 02:30
If a refrigerator is a heat pump that follows the first law of thermodynamics, how much heat was removed from food inside of the refrigerator if it released 380j of energy to the room?
Answers: 1

Physics, 22.06.2019 05:00
Modern physics a photon emitted from an excited hydrogen atom has an energy of 3.02 electron volts. which electron energy-level transition would produce this photon? a. n=1 to n=6 b. n=2 to n=6 c. n=6 to n=1 d. n=6 to n=2 i chose b but the correct answer is d can someone tell me why? and what's the difference?
Answers: 1


Physics, 22.06.2019 20:30
This is a form of winter precipitation. it is frozen precipitation falling as ice pellets. snowflakes melt into raindrops as they pass through a thin layer of warmer air. the raindrops then refreeze into particles of ice when they fall into a layer of sub-freezing air near the surface of the earth. this precipitation is called a) hail. b) rain. c) sleet. d) snow.
Answers: 1
You know the right answer?
Quicklime which is calcium oxide, is made by heathig limestone in a furnace as per the equation :
C...
Questions



Health, 13.11.2019 13:31



Biology, 13.11.2019 13:31

Mathematics, 13.11.2019 13:31

English, 13.11.2019 13:31

Mathematics, 13.11.2019 13:31




Physics, 13.11.2019 13:31


English, 13.11.2019 13:31

Mathematics, 13.11.2019 13:31

Mathematics, 13.11.2019 13:31

Social Studies, 13.11.2019 13:31


Biology, 13.11.2019 13:31