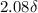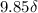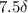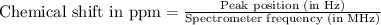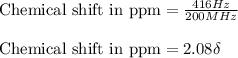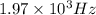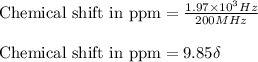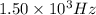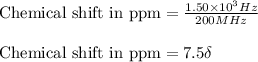Physics, 22.06.2021 23:40 huldalizvega98
The following 1H NMR absorptions were obtained on a spectrometer operating at 200 MHz and are given in Hz downfield from TMS. Convert the absorptions to δ units. a) 416 Hz = δ b) 1.97×103 Hz = δ c) 1.50×103 Hz = δ

Answers: 3
Another question on Physics

Physics, 22.06.2019 10:00
If a stone with an original velocity of 0 is falling from a ledgeand takes 8 seconds to hoybthe ground whays the final velocity of the stone
Answers: 2

Physics, 22.06.2019 13:00
The magnitude of the amount of energy released by burning a fuel source, measured in energy per unit mass, is called its fuel value. note that the fuel value is the negative of the isobaric specific heat of combustion for the fuel. if all the energy obtained from burning 1.23 pounds of butane with a fuel value of 10.85 kcal/g is used to heat 128.0 kg of water at an initial temperature of 18.3 °c, what is the final temperature? note that 1 lb = 453.6 g.
Answers: 3

Physics, 22.06.2019 17:30
Describe cytokinesis in plants and animals. in animals: in plants.
Answers: 2

Physics, 22.06.2019 21:40
Wo small variable-thrust jets are actuated to keep the spacecraft angular velocity about the z-axis constant at ? 0 = 1.16 rad/s as the two telescoping booms are extended from r1 = 1.18 m to r2 = 4.69 m at a constant rate over a period of 124 seconds. the small 19-kg experiment modules at the ends of the booms may be treated as particles, and the mass of the rigid booms is negligible. determine the necessary thrust t for each jet as a function of time where t = 0 is the time when the telescoping action is begun. after you have the general expression for t, answer the questions. show work.
Answers: 1
You know the right answer?
The following 1H NMR absorptions were obtained on a spectrometer operating at 200 MHz and are given...
Questions





History, 24.08.2021 20:50



Mathematics, 24.08.2021 20:50


English, 24.08.2021 20:50


Chemistry, 24.08.2021 20:50

Mathematics, 24.08.2021 20:50



Mathematics, 24.08.2021 20:50


Mathematics, 24.08.2021 20:50

Mathematics, 24.08.2021 20:50