
Two identical cylinders with a movable piston contain 0.7 mol of helium gas at a temperature of 300 K. The temperature of the gas in the first cylinder is increased to 412 K at constant volume by doing work W1 and transferring energy Q1 by heat. The temperature of the gas in the second cylinder is increased to 412 K at constant pressure by doing work W2 while transferring energy Q2 by heat.
Required:
Find ÎEint, 1, Q1, and W1 for the process at constant volume.

Answers: 2
Another question on Physics

Physics, 21.06.2019 14:00
Now that the first three steps have been explained, identify the next three steps, in order. calculate the magnitude of r sum vector components calculate a and b magnitudes calculate the direction of r, calculate x,y components step 4 step 5 step 6
Answers: 1

Physics, 21.06.2019 23:30
After a big snowfall, you take your favorite rocket-powered sled out to a wide field. the field is 195 m across, and you know that your sled accelerates at a rate of 3.65 m/s2 when the rocket is on. how much time will it take the sled to cross the field starting from rest, assuming the rocket is on the whole time?
Answers: 1

Physics, 22.06.2019 14:00
Often called simply "velocity," this is the velocity of an object at a particular moment in time.
Answers: 1

Physics, 22.06.2019 19:30
Which describes increasing the efficiency of energy resource
Answers: 1
You know the right answer?
Two identical cylinders with a movable piston contain 0.7 mol of helium gas at a temperature of 300...
Questions

History, 27.01.2020 22:31



Mathematics, 27.01.2020 22:31



English, 27.01.2020 22:31


Advanced Placement (AP), 27.01.2020 22:31

Health, 27.01.2020 22:31

World Languages, 27.01.2020 22:31





Mathematics, 27.01.2020 22:31




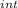 ,₁ = 977.7 J , Q₁ = 977.7 J and W₁ = 0 J
,₁ = 977.7 J , Q₁ = 977.7 J and W₁ = 0 J  = 300 K, T
= 300 K, T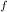 = 412 K, n = 0.7 mol
= 412 K, n = 0.7 mol 

