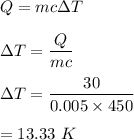Physics, 09.07.2021 06:50 Mw3spartan17
When you hammer a nail into wood, the nail heats up. 30 Joules of energy was absorbed by a 5-g nail as it was hammered into place. How much does the nail's temperature increase (in °C) during this process? (The specific heat capacity of the nail is 450 J/kg-°C, and round to 3 significant digits.

Answers: 3
Another question on Physics

Physics, 22.06.2019 01:30
Ablock of mass 1.5 kg slides down an inclined plane that has an angle of 15. if the inclined plane has no friction and the block starts at a height of 3 m, how much kinetic energy does the block have when it reaches the bottom? acceleration due to gravity is g = 9.8 m/s2. a. 6.8 j b. 50.9 j c. 0 j d. 44.1 j
Answers: 1

Physics, 22.06.2019 08:30
The coefficient of friction is a number that represents the resistance to sliding between two in contact with one another.
Answers: 2

Physics, 22.06.2019 17:40
You throw a baseball directly upward at time =0 at an initial speed of 14.9 m/s. what is the maximum height the ball reaches above where it leaves your hand? ignore air resistance and take =9.80 m/s2.
Answers: 1

Physics, 22.06.2019 18:30
Anonzero net force acts on a particle and does work. which one of the following statements is true? the kinetic energy of the particle changes, but the speed of the particle does not change. the kinetic energy of the particle does not change, but the speed of the particle does change. the kinetic energy of the particle changes, but the velocity of the particle does not change. the kinetic energy and the speed of the particle change, but the velocity of the particle does not change. the kinetic energy, speed, and velocity of the particle change.
Answers: 1
You know the right answer?
When you hammer a nail into wood, the nail heats up. 30 Joules of energy was absorbed by a 5-g nail...
Questions

Mathematics, 30.10.2020 07:20

History, 30.10.2020 07:20

Chemistry, 30.10.2020 07:20



English, 30.10.2020 07:20




Chemistry, 30.10.2020 07:20

Mathematics, 30.10.2020 07:20

Health, 30.10.2020 07:20

Mathematics, 30.10.2020 07:20



Mathematics, 30.10.2020 07:20



Computers and Technology, 30.10.2020 07:20