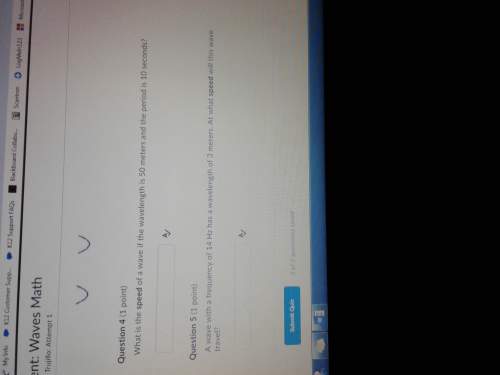
Physics, 11.07.2021 16:50 hgdthbgjnb5604
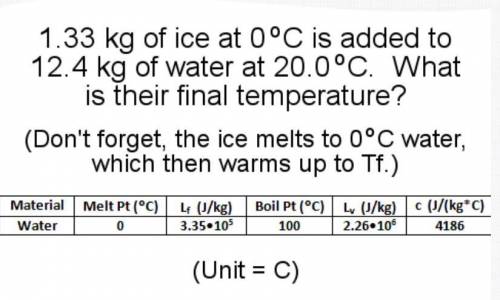
1.33 kg of ice at 0 degrees C is added to 12.4 kg of water at 20.0 C. What is their final temperature? (Don't forget, the ice melts to O degrees C water, which then warms up to T f.)

Answers: 2
Another question on Physics

Physics, 22.06.2019 04:10
Calculate the work done by an external agent during an isothermal compression of 1.00 mol of oxygen from a volume of 22.4 l at 10∘c and 1.0 atm pressure to 16.8l
Answers: 2

Physics, 22.06.2019 05:50
Acylinder with a movable piston contains 11.7 moles of a monatomic ideal gas at a pressure of 1.32×10^5 pa. the gas is initially at a temperature of 300 k. an electric heater adds 43200 j of energy into the gas while the piston moves in such a way that the pressure remains constant. cp=20.79 j k^−1 mol^−1 for a monatomic ideal gas, and that the number of gas molecules is equal to avogadro's number (6.022×10^23) times the number of moles of the gas. (a) what is the temperature of the gas after the energy is added? (b) what is the change in volume of the gas? (c) how much work is done by the gas during this process?
Answers: 3

Physics, 22.06.2019 16:20
What is the mass of the water that is being heated? it requires 2,500 joules to raise a certain amount of water (c = 4.186 jig c) from 20.0°c to 60.0°c. o 159 o 40 g o 63 g o 80 g
Answers: 2

Physics, 22.06.2019 16:30
He latent heat of vaporization for ethyl alcohol is 854 j/g. the amount of energy, rounded to the nearest whole number, needed to change 5.20 grams of ethyl alcohol from a liquid to a gas is
Answers: 2
You know the right answer?
1.33 kg of ice at 0 degrees C is added to 12.4 kg of water at 20.0 C. What is their final temperatur...
Questions

Mathematics, 11.10.2020 21:01


Advanced Placement (AP), 11.10.2020 21:01

Mathematics, 11.10.2020 21:01

Mathematics, 11.10.2020 21:01


History, 11.10.2020 21:01

Mathematics, 11.10.2020 21:01


Mathematics, 11.10.2020 21:01

Mathematics, 11.10.2020 21:01


Mathematics, 11.10.2020 21:01

Mathematics, 11.10.2020 21:01


Mathematics, 11.10.2020 21:01


English, 11.10.2020 21:01