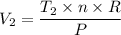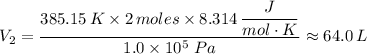Physics, 11.07.2021 18:00 puppylover72
When 2 moles of helium gas expand at a constant pressure p= 1.0×10^5 pascals, the temperature increase from 2 °c to 112 °c. If the initial volume of the gas was 45 liters. Cp= 20.8j/mol. K, Cv= 12.6j/mol. K. Determine i. The work done W by the gas as it exands

Answers: 3
Another question on Physics

Physics, 21.06.2019 17:10
An air-standard stirling cycle operates with a maximum pressure of 600 psia and a minimum pressure of 10 psia. the maximum volume of the air is 10 times the minimum volume. the temperature during the heat rejection process is 100°f. calculate the specific heat added to and rejected by this cycle, as well as the net specific work produced by the cycle. use constant specific heats at room temperature. the properties of air at room temperature are r
Answers: 2

Physics, 21.06.2019 18:40
The intensity, or loudness, of a sound can be measured in decibels (db), according to the equation , where i is the intensity of a given sound and is the threshold of hearing intensity. what is the intensity, in decibels, [i(db)], when ? use a calculator. round to the nearest whole number. 8 9 19 80
Answers: 1

Physics, 22.06.2019 02:30
Which type of bond is found between the atoms of a molecule? a. metallic b. delocalized electron c. covalent d. ionic
Answers: 1

Physics, 22.06.2019 18:00
Wind and moving water provide energy. question 1 options: chemical mechanical thermal none of the above
Answers: 1
You know the right answer?
When 2 moles of helium gas expand at a constant pressure p= 1.0×10^5 pascals, the temperature increa...
Questions

Business, 10.11.2020 01:40






Biology, 10.11.2020 01:40

Mathematics, 10.11.2020 01:40

Mathematics, 10.11.2020 01:40

Social Studies, 10.11.2020 01:40

History, 10.11.2020 01:40

History, 10.11.2020 01:40


History, 10.11.2020 01:40

Mathematics, 10.11.2020 01:40

Mathematics, 10.11.2020 01:40




History, 10.11.2020 01:40