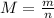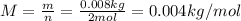Physics, 19.07.2021 06:00 fatimahellis33
A monatomic gas is measured to have an average speed of 1477 m/s. If the
total amount of the gas is 2 mol (which equates to a mass of 0.008 kg), what
is the approximate temperature of the gas? (Recall that the equation for
kinetic energy due to translation in a gas is KEtranslational = 1 mv2 = 3 nRT,
2
and R = 8.31 J/(mol-K).)
2

Answers: 2
Another question on Physics

Physics, 21.06.2019 17:00
How much work is done lifting a 9.10 kg box straight up onto a shelf that is 1.80 m high?
Answers: 1

Physics, 22.06.2019 03:00
Standing side by side ,you and a friend step off a bridge at different times and fall for 1.6s to the water below.your friend goes first,and you follow after he has dropped a distance of 2.0m.when your friend hits the water,is the seperation between the two of you 2.0m or more than 2.0m? verify your answer with a calculation.
Answers: 2


Physics, 22.06.2019 15:50
Ryan is examining the energy of the particles in a bar of gold. what is ryan most likely studying?
Answers: 1
You know the right answer?
A monatomic gas is measured to have an average speed of 1477 m/s. If the
total amount of the gas is...
Questions



Social Studies, 28.07.2019 21:20

Biology, 28.07.2019 21:20

Biology, 28.07.2019 21:20


Biology, 28.07.2019 21:20

Biology, 28.07.2019 21:20

Social Studies, 28.07.2019 21:20

History, 28.07.2019 21:20

Health, 28.07.2019 21:20


Geography, 28.07.2019 21:20


Mathematics, 28.07.2019 21:20



Biology, 28.07.2019 21:20

Mathematics, 28.07.2019 21:20

History, 28.07.2019 21:20

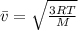 (1)
(1)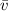 : is the average speed = 1477 m/s
: is the average speed = 1477 m/s