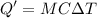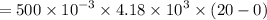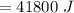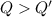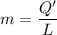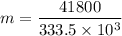Answers: 2
Another question on Physics


Physics, 22.06.2019 09:30
True or false graphs must include scales that increase by the same amount
Answers: 1

Physics, 22.06.2019 10:00
Which atomic model was proposed as a result of j. j. thomson’s work?
Answers: 1

Physics, 22.06.2019 10:30
The uniform slender bar rests on a smooth horizon- tal surface when a force f is applied normal to the bar at point a. point a is observed to have an initial acceleration aa of 20 m/s2, and the bar has a corre- sponding angular acceleration of 18 rad /s2. deter- mine the distance b.
Answers: 1
You know the right answer?
A 200 g piece of ice at 0°C is placed in 500 g of water at 20°C. The system is in a container of neg...
Questions

Mathematics, 04.02.2020 09:55

Chemistry, 04.02.2020 09:55





Mathematics, 04.02.2020 09:55



Biology, 04.02.2020 09:55



Spanish, 04.02.2020 09:55


Mathematics, 04.02.2020 09:55





History, 04.02.2020 09:55

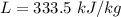
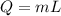
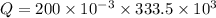
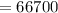 J
J