
Physics, 21.09.2021 03:10 lilacastro
Water at 23.0 °C is sprayed onto 0.140 kg of molten gold at 1063 °C (its melting point). The water boils away, forming steam at 100.0 °C and leaving solid gold at 1063 °C. What is the minimum mass of water that must be used?
Could someone please checking my working out? My answer is incorrect and I'm not sure why.
The answer should be in kg
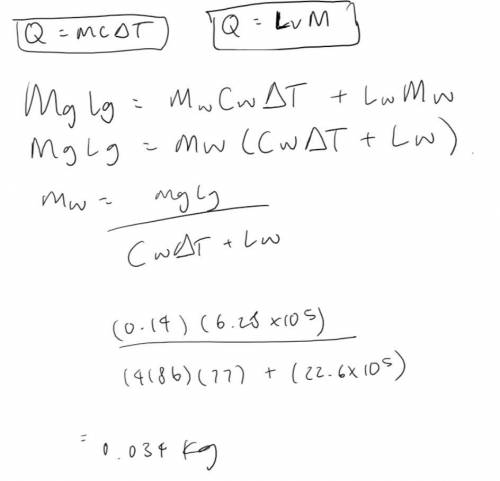

Answers: 2
Another question on Physics

Physics, 22.06.2019 07:30
Carbon-14 is a radioactive element that undergoes beta decay. which force is responsible for allowing carbon-14 to become stable? electromagnetic gravitational weak nuclear strong nuclear
Answers: 2

Physics, 22.06.2019 07:40
Astudent creates a model of a closed ecosystem by filling a glass tank half full with water, then adding 10 snails and two small aquatic plants. the next day, all the snails are dead. what is the most likely cause of their death?
Answers: 3

Physics, 22.06.2019 14:30
Which compound is held together by the electrostatic force between two ions? a. co2 b. cci4 c. h2s d. mgf2
Answers: 1

Physics, 22.06.2019 15:30
1kg of water needs to recieve 4200j of energy to go from 90 degrees to 91 degrees. what is the specific heat capacity of water? what are the units?
Answers: 1
You know the right answer?
Water at 23.0 °C is sprayed onto 0.140 kg of molten gold at 1063 °C (its melting point). The water b...
Questions

History, 21.07.2019 06:20



History, 21.07.2019 06:20


Biology, 21.07.2019 06:20

Mathematics, 21.07.2019 06:20




Social Studies, 21.07.2019 06:20

Biology, 21.07.2019 06:20

Business, 21.07.2019 06:20

Biology, 21.07.2019 06:20



Health, 21.07.2019 06:20


Social Studies, 21.07.2019 06:20



