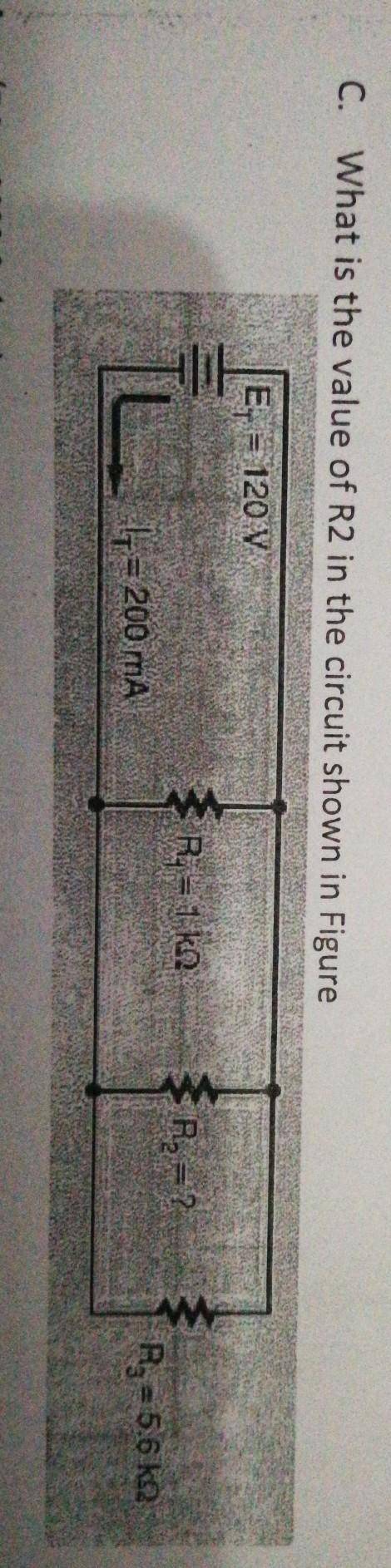
What is the value of R2 in the circuit shown below
...

Answers: 2
Another question on Physics

Physics, 22.06.2019 08:40
When temperature of a substance increases, of its particles increase resulting in more between particles. real world applications of thermal expansion absolute zero (-273 degrees c) is the lowest possible temperature on the kelvin scale. it is a measure of where a substance has no kinetic energy. heat is a process of the . energy flows spontaneously from a object to a until equilibrium is reached. heat is measured in heat is transferred throughis a method of heat transfer that occurs between particles of matter that are in direct contact with each other. are poor conductors that impede / reduce the rate of heat transfer. is a method of heat transfer that occurs in fluids (non-solids) by means of currents. real world application of convectiona method of heat transfer through electromagnetic waves. when energy moves from one object to another inside a closed system, no energy is or in the system in the transaction. systems will spontaneously move to a state of organized energy or high disorder over time called
Answers: 2

Physics, 22.06.2019 14:00
Estimate the change in the gibbs energy and molar gibbs energy of 1.0dm3 of octane when the pressure acting on it is increased from 1.0 atm to 100 atm. the mass density of octane is 0.703 g cm−3
Answers: 3

Physics, 22.06.2019 16:00
From the perspective of an employee that effective channeling of work related information and concerns
Answers: 1

Physics, 23.06.2019 08:30
[05.02] what is the best explanation of the kinetic molecular theory as it relates to the energy of the molecules in the states of matter? (1 point) the molecules in a gas have greater kinetic energy than those in a liquid, which in turn have greater kinetic energy than the molecules in a solid. kinetic energy cannot be created nor destroyed, and, as a result, all states of matter have the same kinetic energy. the molecules in a gas have greater kinetic energy; therefore, they are more tightly packed than the molecules in either a liquid or a solid. the molecules in gases and liquids, in constant motion, have greater kinetic energy than those in a solid, which do not move or vibrate because their position is fixed.
Answers: 2
You know the right answer?
Questions

Mathematics, 24.11.2020 01:00


Mathematics, 24.11.2020 01:00

History, 24.11.2020 01:00

Mathematics, 24.11.2020 01:00

Mathematics, 24.11.2020 01:00



Business, 24.11.2020 01:00

Mathematics, 24.11.2020 01:00

Arts, 24.11.2020 01:00

History, 24.11.2020 01:00


English, 24.11.2020 01:00