
Physics, 27.10.2021 21:10 kelchalyssa
How would these two particle diagrams be classified?
A-) Both are pure substances each containing one type of compound
B-)Both are pure substances containing a mixture of elements
C-) Both are mixtures containing different types of compounds
D-) Both are mixtures containing more than one element
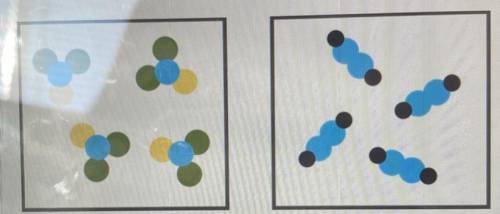

Answers: 2
Another question on Physics

Physics, 22.06.2019 00:30
Which of the following elements is in the same period as phosphorus? a. carbon c. nitrogen b. magnesium d. oxygen select the best answer from the choices provided a b c d
Answers: 1

Physics, 22.06.2019 00:30
There are weak or strong attractive forces between atoms of liquids with a high viscosity
Answers: 3

Physics, 22.06.2019 05:50
High schoolphysics 5+3 pts a neon light consists of a glass tube with metal wires at each end. when connected to a high-voltage source, the gas glows. if a fly lands on the glass tube, what will most likely happen? a) the fly will not feel a shock because the glass conducts any free electrons back into the gas. b) electrons will flow directly from the metal wires along the glass and shock the fly. c) the fly will not feel a shock because the glass insulates it from the electrons in the gas and the metal. d) electrons that are moving through the gas will be conducted through the glass and shock the fly.
Answers: 3

Physics, 22.06.2019 15:20
Your science teacher brings in a speaker to talk to your class about climate change. during the session, students ask a few questions. which questions are related to the current evidence on climate change
Answers: 3
You know the right answer?
How would these two particle diagrams be classified?
A-) Both are pure substances each containing...
Questions


History, 04.08.2019 16:30

Mathematics, 04.08.2019 16:30

English, 04.08.2019 16:30

Spanish, 04.08.2019 16:30


History, 04.08.2019 16:30


Spanish, 04.08.2019 16:30





History, 04.08.2019 16:30

Social Studies, 04.08.2019 16:30


History, 04.08.2019 16:30

Business, 04.08.2019 16:30

Social Studies, 04.08.2019 16:30

Biology, 04.08.2019 16:30



