
Physics, 27.10.2021 23:40 carnations
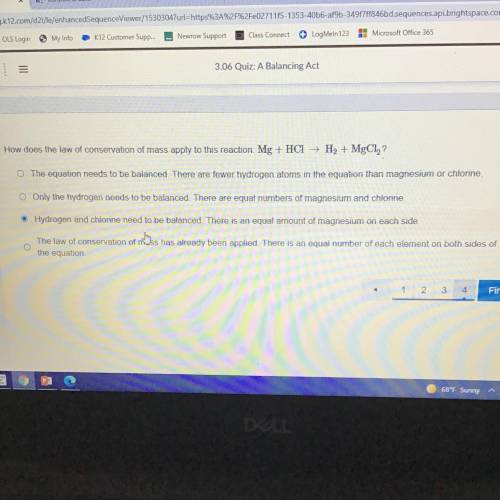
How does the law of conservation of mass apply to this reaction: Mg + HCI + H2 MgClz?
O The equation needs to be balanced. There are fewer hydrogen atoms in the equation than magnesium or chlorine.
© Only the hydrogen needs to be balanced. There are equal numbers of magnesium and chiorine.
Hydrogen and chlorine need to be balanced. There is an equal amount of magnesium on each side
©
The
law of conservation of nÖss has already been
the equation.
applied. There is an equal number of each element on both sides of

Answers: 3
Another question on Physics

Physics, 21.06.2019 17:30
Abaseball picther can throw a ball at about 90 miles per hour, v = 40 m/s, on solid rock ground. we now put him into space. when he throws the ball, which weighs mb = 0.15 kg moves away from himat the same speed. how long does it take the ball thrown in space to travel the 30 meters to the batter? he weighs mp = 100kg
Answers: 1

Physics, 22.06.2019 01:00
Avehicle has an oil leak that is causing the entire oil pan to be wet, but inspection reveals no exact source after cleaning off the oil residue. technician a says to install a fluorescent dye in the crankcase and operate the engine, then re-inspect for leaks with a special light (black light). technician b says the oil leak may be coming from a source at the top of the engine, such as a valve cover gasket. who is correct?
Answers: 1

Physics, 22.06.2019 14:00
What is the force that opposes motion and works against the downward pull? a) friction b) gravity c) weight d) acceleration
Answers: 1

Physics, 22.06.2019 16:00
Which composition of water moves to begin a deepwater current?
Answers: 2
You know the right answer?
How does the law of conservation of mass apply to this reaction: Mg + HCI + H2 MgClz?
O The equati...
Questions



History, 14.01.2020 13:31


Social Studies, 14.01.2020 13:31


Chemistry, 14.01.2020 13:31


History, 14.01.2020 13:31

Biology, 14.01.2020 13:31

Social Studies, 14.01.2020 13:31


History, 14.01.2020 13:31

Chemistry, 14.01.2020 13:31





Mathematics, 14.01.2020 13:31



