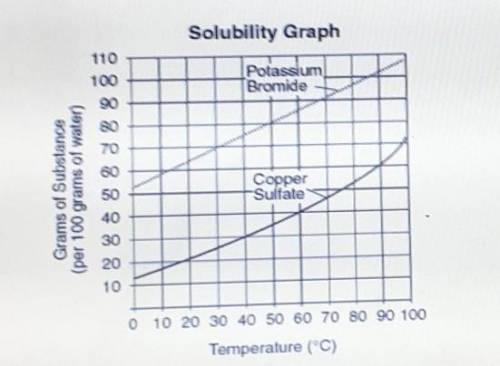
Question: What is the relationship between solubilty and the temperature of The solvent
...

Physics, 25.11.2021 07:10 robertss403
Question: What is the relationship between solubilty and the temperature of The solvent

Answers: 1
Another question on Physics

Physics, 22.06.2019 08:00
If the force applied to an object remains constant, is more power needed for the object to move faster ? explain
Answers: 3

Physics, 22.06.2019 08:20
At an oceanside nuclear power plant, seawater is used as part of the cooling system. this raises the temperature of the water that is discharged back into the ocean. the amount that the water temperature is raised has a uniform distribution over the interval from 10° to 25° c. what is the standard deviation of the temperature increase?
Answers: 1

Physics, 22.06.2019 15:00
10 points! will mark brainiest! in a heat engine if 1,000 j of heat enters the system and the piston does 500 j of work, what is the final internal energy of the system if the initial energy was 2,000 j 1: write the equation 2: list out your known variables 3: plug the numbers into the equations 4: solve 5: write your solution statement that includes initial energy and final energy added you so much!
Answers: 3

Physics, 22.06.2019 16:00
The electric potential v is constant everywhere within a certain region of space. which statement below is true? the choices are: the electric field is also constant (but not zero) within the region. a charged particle placed within the region will experience an electric force. the electric field is zero everywhere within the region. the electric field varies from place to place within the region.
Answers: 2
You know the right answer?
Questions

Biology, 10.04.2020 00:58

Mathematics, 10.04.2020 00:58



Mathematics, 10.04.2020 00:58

History, 10.04.2020 00:58

English, 10.04.2020 00:58


Spanish, 10.04.2020 00:58

Mathematics, 10.04.2020 00:58

Mathematics, 10.04.2020 00:58



Mathematics, 10.04.2020 00:59


Biology, 10.04.2020 00:59

Computers and Technology, 10.04.2020 00:59





