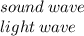Answers: 2
Another question on Physics

Physics, 22.06.2019 03:30
Imagine you are riding on a yacht in the ocean and traveling at 20 mph. you then hit a golf ball at 100 mph from the deck of the yacht. you see the ball move away from you at 100mph, while a person standing on a near by beach would observe your golf ball traveling at 120 mph (20 mph + 100 mph). now imagine you are aboard the hermes spacecraft traveling at 0.1c (1/10 the speed of light) past mars and shine a laser from the front of the ship. you would see the light traveling at c (the speed of light) away from your ship. according to einstein’s special relativity, how fast will a person on mars observe the light to be traveling? a) 0.1c (1/10 the speed of light) b) c (the speed of light) c)1.1c (c+0.1c)
Answers: 1

Physics, 22.06.2019 22:00
Ultraviolet radiation is dangerous because it has a high enough energy to damage skin cells. what is the best explanation of the relationship between wavelength and wave energy? a) wave energy is related only to frequency, not wavelength. b) longer wavelengths have lower frequencies but higher energy. c) the shorter the wavelength, the lower the frequency and the higher the energy. d) the shorter the wavelength, the higher the frequency and the higher the energy.
Answers: 3

Physics, 23.06.2019 05:30
Aman jogs at a velocity of 2.5 m/s west for 1,200 s. he then walks at a velocity 1.0 m/s east for 500 s and then stops to rest . what is the displacement of man when he stops to rest?
Answers: 3

Physics, 23.06.2019 09:30
How many milliliters of water at 23 °c with a density of 1.00 g/ml must be mixed with 180 ml (about 6 oz) of coffee at 95 °c so that the resulting combination will have a temperature of 60 °c? assume that coffee and water have the same density and the same specific heat. how much will the temperature of a cup (180 g) of coffee at 95 °c be reduced when a 45 g silver spoon (specific heat 0.24 j/g °c) at 25 °c is placed in the coffee and the two are allowed to reach the same temperature? assume that the coffee has the same density and specific heat as water. a 45-g aluminum spoon (specific heat 0.88 j/g °c) at 24 °c is placed in 180 ml (180 g) of coffee at 85 °c and the temperature of the two become equal. (a) what is the final temperature when the two become equal? assume that coffee has the same specific heat as water. (b) the first time a student solved this problem she got an answer of 88 °c. explain why this is clearly an incorrect answer.
Answers: 1
You know the right answer?
Which two types of waves require matter in order to travel...
Questions


Arts, 27.05.2021 20:50

Mathematics, 27.05.2021 20:50

Mathematics, 27.05.2021 20:50




Mathematics, 27.05.2021 20:50

Mathematics, 27.05.2021 20:50

Mathematics, 27.05.2021 20:50

English, 27.05.2021 20:50

Mathematics, 27.05.2021 20:50

English, 27.05.2021 20:50

Mathematics, 27.05.2021 20:50



English, 27.05.2021 20:50

Mathematics, 27.05.2021 20:50