
Which statement best explains why the specific heat of water is higher than the specific heat of most other substances? a) due to its polarity and hydrogen bonding water can absorb heat without a significant temperature change. b) due to the ionic bonding in liquid water, it takes more heat energy to reach boiling. c) because water is a covalent compound, it takes a lot of energy to change its kinetic energy. d) water has a higher density value than most substances and requires more energy to change the temperature.

Answers: 1
Another question on Physics


Physics, 22.06.2019 14:30
70 give a real life example showing how sensory neurons work with the motor neurons
Answers: 2

Physics, 22.06.2019 15:00
Lightning is an example of what phenomenon? a release of a large amount of energy an absorption of a large amount of energy a natural electric circuit a natural electric current
Answers: 1

Physics, 22.06.2019 15:20
Aphoton is absorbed by an electron that is in the n = 3 state of a hydrogen atom, causing the hydrogen atom to become ionized. very far away from the nucleus, the released electron has a velocity of 750,000 m/s. what was the wavelength of the absorbed photon?
Answers: 2
You know the right answer?
Which statement best explains why the specific heat of water is higher than the specific heat of mos...
Questions


Arts, 03.03.2021 19:40

Biology, 03.03.2021 19:40


Mathematics, 03.03.2021 19:40


Arts, 03.03.2021 19:40



English, 03.03.2021 19:40


Mathematics, 03.03.2021 19:40


Biology, 03.03.2021 19:40

Mathematics, 03.03.2021 19:40

Mathematics, 03.03.2021 19:40

Mathematics, 03.03.2021 19:40

Mathematics, 03.03.2021 19:40


Biology, 03.03.2021 19:40

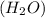 is formed of two hydrogen atoms and one oxygen atom. The atoms are have strong hydrogen bond. Also, water is a polar molecule. there is uneven distribution of density of electrons. This makes the bond even more stronger between the hydrogen atoms and oxygen. Hence, the heat required to raise the temperature of water is high compared to specific heat of other substances.
is formed of two hydrogen atoms and one oxygen atom. The atoms are have strong hydrogen bond. Also, water is a polar molecule. there is uneven distribution of density of electrons. This makes the bond even more stronger between the hydrogen atoms and oxygen. Hence, the heat required to raise the temperature of water is high compared to specific heat of other substances. 

