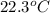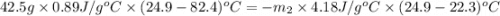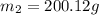Physics, 29.07.2019 02:00 christabell0303
A42.5 g piece of aluminum (which has a molar heat capacity of 24.03 j/ocmol) is heated to 82.4oc and dropped into a calorimeter containing water (specific heat capacity of water is 4.18 j/goc) initially at 22.3oc. the final temperature of the water is 24.9oc. calculate the mass of water in the calorimeter. ignore significant figures for this problem.

Answers: 1
Another question on Physics

Physics, 22.06.2019 08:30
Does anyone know how to solve this problem? i really need . i made an attempt but i just cant get it. a metal rod is 25.000 cm long at 25.0 degrees celsius. when heated to 102.0 degrees celsius, it is 25.054 cm long. what is the coefficient of linear expansion for this metal.
Answers: 3


Physics, 22.06.2019 09:30
Need asap ‼️ 20 pts which gravitational force field diagram is drawn correctly? (answers in pictures below)
Answers: 1

Physics, 22.06.2019 10:30
Astone weighing 1.5 kilograms is resting on a rock at a height of 20 meters above the ground. the stone rolls down 10 meters and comes to rest on a patch of moss. the gravitational potential energy of the stone on the moss is joules.
Answers: 1
You know the right answer?
A42.5 g piece of aluminum (which has a molar heat capacity of 24.03 j/ocmol) is heated to 82.4oc and...
Questions

Health, 29.01.2020 03:09





Chemistry, 29.01.2020 03:09



Business, 29.01.2020 03:09


History, 29.01.2020 03:09





Computers and Technology, 29.01.2020 03:09

English, 29.01.2020 03:09


Spanish, 29.01.2020 03:09

Mathematics, 29.01.2020 03:09

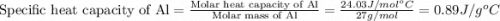
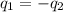
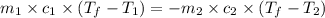
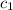 = specific heat of aluminum =
= specific heat of aluminum = 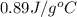
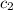 = specific heat of water =
= specific heat of water = 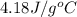
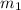 = mass of Al = 42.5 g
= mass of Al = 42.5 g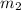 = mass of water = ?
= mass of water = ?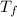 = final temperature of water =
= final temperature of water = 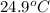
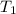 = initial temperature of Al =
= initial temperature of Al = 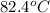
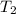 = initial temperature of water =
= initial temperature of water =